ট্যানটালাম: সংশোধিত সংস্করণের মধ্যে পার্থক্য
Shariful iea (আলোচনা | অবদান) সম্পাদনা সারাংশ নেই |
সম্পাদনা সারাংশ নেই |
||
| ১ নং লাইন: | ১ নং লাইন: | ||
{{Elementbox_header | number= |
{{Elementbox_header | number=৭৩ | symbol=Ta | name=ট্যানটালাম | left=[[হ্যাফনিয়াম]] | right=[[টাংস্টেন]] | above=[[নিওবিয়াম|Nb]] | below=[[ডুবনিয়াম|Db]] | color1=#ffc0c0 | color2=black }} |
||
{{Elementbox_series | [[অবস্থান্তর ধাতু]] }} |
{{Elementbox_series | [[অবস্থান্তর ধাতু]] }} |
||
{{Elementbox_groupperiodblock | গ্রুপ= |
{{Elementbox_groupperiodblock | গ্রুপ=৫ | পর্যায়=৬ | ব্লক=ডি }} |
||
{{Elementbox_appearance_img | Ta,73| |
{{Elementbox_appearance_img | Ta,73| ধূসর নীল }} |
||
{{Elementbox_atomicmass_gpm | [[1 E-25 kg| |
{{Elementbox_atomicmass_gpm | [[1 E-25 kg|১৮০.৯৪৭৮৮]][[List of elements by atomic mass|(২)]] }} |
||
{{Elementbox_econfig | [[[জেনন|Xe]]] |
{{Elementbox_econfig | [[[জেনন|Xe]]] ৪এফ<sup>১৪</sup> ৫ডি<sup>৩</sup> ৬এস<sup>২</sup> }} |
||
{{Elementbox_epershell | |
{{Elementbox_epershell | ২, ৮, ১৮, ৩২, ১১, ২ }} |
||
{{Elementbox_section_physicalprop | color1=#ffc0c0 | color2=black }} |
{{Elementbox_section_physicalprop | color1=#ffc0c0 | color2=black }} |
||
{{Elementbox_phase | [[কঠিন]] }} |
{{Elementbox_phase | [[কঠিন]] }} |
||
{{Elementbox_density_gpcm3nrt | |
{{Elementbox_density_gpcm3nrt | ১৬.৬৯ }} |
||
{{Elementbox_densityliq_gpcm3mp | |
{{Elementbox_densityliq_gpcm3mp | ১৫ }} |
||
{{Elementbox_meltingpoint | k= |
{{Elementbox_meltingpoint | k=৩২৯০ | c=৩০১৭ | f=৫৪৬৩ }} |
||
{{Elementbox_boilingpoint | k= |
{{Elementbox_boilingpoint | k=৫৭৩১ | c=৫৪৫৮ | f=৯৮৫৬ }} |
||
{{Elementbox_heatfusion_kjpmol | |
{{Elementbox_heatfusion_kjpmol | ৩৬.৫৭ }} |
||
{{Elementbox_heatvaporiz_kjpmol | |
{{Elementbox_heatvaporiz_kjpmol | ৭৩২.৮ }} |
||
{{Elementbox_heatcapacity_jpmolkat25 | |
{{Elementbox_heatcapacity_jpmolkat25 | ২৫.৩৬ }} |
||
{{Elementbox_vaporpressure_katpa | |
{{Elementbox_vaporpressure_katpa | ৩২৯৭ | ৩৫৯৭ | ৩৯৫৭ | ৪৩৯৫ | ৪৯৩৯ | ৫৬৩৪ | comment= }} |
||
{{Elementbox_section_atomicprop | color1=#ffc0c0 | color2=black }} |
{{Elementbox_section_atomicprop | color1=#ffc0c0 | color2=black }} |
||
{{Elementbox_crystalstruct | |
{{Elementbox_crystalstruct | ঘনক কেন্দ্রিক }} |
||
{{Elementbox_oxistates | 5<br />( |
{{Elementbox_oxistates | 5<br />(হালকা [[অম্ল|আম্লিক]] অক্সাইড) }} |
||
{{Elementbox_electroneg_pauling | |
{{Elementbox_electroneg_pauling | ১.৫ }} |
||
{{Elementbox_ionizationenergies2 | |
{{Elementbox_ionizationenergies2 | ৭৬১ | ১৫০০ }} |
||
{{Elementbox_atomicradius_pm | [[1 E-10 m| |
{{Elementbox_atomicradius_pm | [[1 E-10 m|১৪৫]] }} |
||
{{Elementbox_atomicradiuscalc_pm | [[1 E-10 m| |
{{Elementbox_atomicradiuscalc_pm | [[1 E-10 m|২০০]] }} |
||
{{Elementbox_covalentradius_pm | [[1 E-10 m| |
{{Elementbox_covalentradius_pm | [[1 E-10 m|১৩৮]] }} |
||
{{Elementbox_section_miscellaneous | color1=#ffc0c0 | color2=black }} |
{{Elementbox_section_miscellaneous | color1=#ffc0c0 | color2=black }} |
||
{{Elementbox_magnetic | |
{{Elementbox_magnetic | কোন তথ্য নেই }} |
||
{{Elementbox_eresist_ohmmat20 | |
{{Elementbox_eresist_ohmmat20 | ১৩১ n}} |
||
{{Elementbox_thermalcond_wpmkat300k | |
{{Elementbox_thermalcond_wpmkat300k | ৫৭.৫ }} |
||
{{Elementbox_thermalexpansion_umpmkat25 | |
{{Elementbox_thermalexpansion_umpmkat25 | ৬.৩ }} |
||
{{Elementbox_speedofsound_rodmpsat20 | |
{{Elementbox_speedofsound_rodmpsat20 | ৩৪০০ }} |
||
{{Elementbox_youngsmodulus_gpa | |
{{Elementbox_youngsmodulus_gpa | ১৮৬ }} |
||
{{Elementbox_shearmodulus_gpa | |
{{Elementbox_shearmodulus_gpa | ৬৯ }} |
||
{{Elementbox_bulkmodulus_gpa | |
{{Elementbox_bulkmodulus_gpa | ২০০ }} |
||
{{Elementbox_poissonratio | |
{{Elementbox_poissonratio | ০.৩৪ }} |
||
{{Elementbox_mohshardness | |
{{Elementbox_mohshardness | ৬.৫ }} |
||
{{Elementbox_vickershardness_mpa | |
{{Elementbox_vickershardness_mpa | ৮৭৩ }} |
||
{{Elementbox_brinellhardness_mpa | |
{{Elementbox_brinellhardness_mpa | ৮০০ }} |
||
{{Elementbox_cas_number | |
{{Elementbox_cas_number | ৭৪৪০-২৫-৭ }} |
||
{{Elementbox_isotopes_begin | isotopesof=tantalum | color1=#ffc0c0 | color2=black }} |
{{Elementbox_isotopes_begin | isotopesof=tantalum | color1=#ffc0c0 | color2=black }} |
||
{{Elementbox_isotopes_decay | mn=177 | sym=Ta |
{{Elementbox_isotopes_decay | mn=177 | sym=Ta |
||
| ৬৮ নং লাইন: | ৬৮ নং লাইন: | ||
{{Elementbox_isotopes_end}} |
{{Elementbox_isotopes_end}} |
||
{{Elementbox_footer | color1=#ffc0c0 | color2=black }} |
{{Elementbox_footer | color1=#ffc0c0 | color2=black }} |
||
'''ট্যানটালাম''' হল একটি [[মৌলিক পদার্থ]], এর [[প্রতীক (রসায়ন) | প্রতীক]] '''Ta''' এবং [[পারমাণবিক সংখ্যা]] ৭৩। আগে এর নাম ছিল '' ট্যানটালিয়াম '', গ্রীক পৌরাণিক কাহিনীর খলনায়ক '' [[ট্যান্টালাস|ট্যান্টালাসের]] '' নামে এখন এর নামকরণ করা হয়েছে।<ref>[[Euripides]], ''[[Orestes (play)|Orestes]]''</ref> ট্যানটালাম একটি বিরল, শক্ত, ধূসর নীল, [[দীপ্তি (খনিজ পদার্থ) |চকচকে)] [[অবস্থান্তর ধাতু]], এটি ভীষণভাবে ক্ষয়-প্রতিরোধী। এটি [[উচ্চতাপ সহনশীল ধাতু]] দলের একটি অংশ, যেগুলি |
|||
'''ট্যানটালাম''' পর্যায় সারণীর ৭৩ নম্বর [[মৌল]], [[প্রতিক]] Ta। |
|||
সঙ্কর ধাতু তৈরিতে ক্ষুদ্র উপাদান হিসাবে ব্যাপকভাবে ব্যবহৃত হয়। ট্যানটালাম রাসায়নিকভাবে নিষ্ক্রিয় হওয়ায়, পরীক্ষাগারে এটি একটি অত্যন্ত প্রয়োজনীয় মূল্যবান পদার্থ এবং এটিকে [[প্লাটিনাম|প্লাটিনামের]] বিকল্প হিসেবে ব্যবহার করা হয়। [[ট্যানটালাম ক্যাপাসিটার]] হিসেবে এটি প্রধানত ব্যবহার হয় [[ইলেকট্রন বিজ্ঞান| বৈদ্যুতিন]] সরঞ্জামগুলিতে, যার মধ্যে আছে [[মোবাইল ফোন]], [[ডিভিডি প্লেয়ার]], [[ভিডিও গেইম কনসোল]] এবং [[ব্যক্তিগত কম্পিউটার]]। |
|||
রাসায়নিকভাবে অনুরূপ [[নাইওবিয়াম|নাইওবিয়ামের]] সাথে, ট্যানটালামকে সর্বদা [[ট্যানটালাইট]], [[কলম্বাইট]] এবং [[কোল্টান]] [[খনিজ]] গ্রুপে (পৃথক খনিজ প্রজাতি হিসাবে স্বীকৃত না হলেও কলম্বাইট এবং ট্যানটালাইটের মিশ্রণ) দেখা যায়।<ref name="mindat.org">{{cite web|url=http://www.mindat.org|title=Mindat.org - Mines, Minerals and More|website=www.mindat.org}}</ref> Tantalum is considered a [[Technology-critical element|technology-critical element]]. |
|||
==ইতিহাস== |
|||
{{নিবিড় পর্যায় সারণী}} |
|||
১৮০২ সালে ট্যানটালাম আবিষ্কার করেছিলেন [[সুইডেন|সুইডেনের]] [[অ্যান্ডার্স একেবার্গ]], সুইডেন এবং ফিনল্যান্ডের দুটি খনিজ নমুনায় তিনি এর সন্ধান পান।<ref>{{cite journal | journal = Journal of Natural Philosophy, Chemistry, and the Arts | pages = 251–255 | volume = 3 | year = 1802| first = Anders | last = Ekeberg | title = Of the Properties of the Earth Yttria, compared with those of Glucine; of Fossils, in which the first of these Earths in contained; and of the Discovery of a metallic Nature (Tantalium) | url = https://www.biodiversitylibrary.org/item/15589#page/265/mode/1up}}</ref><ref>{{cite journal | journal = Kungliga Svenska Vetenskapsakademiens Handlingar |year = 1802 | pages = 68–83 | volume = 23| first = Anders | last = Ekeberg | title = Uplysning om Ytterjorden egenskaper, i synnerhet i aemforelse med Berylljorden:om de Fossilier, havari förstnemnde jord innehales, samt om en ny uptäckt kropp af metallik natur | url = https://archive.org/details/kungligasvenskav2231kung}}</ref> এর এক বছর আগে, [[চার্লস হ্যাচেট]] [[কলম্বিয়াম]] (এখন নাইওবিয়াম) আবিষ্কার করেছিলেন,<ref>{{cite journal|title = Charles Hatchett FRS (1765–1847), Chemist and Discoverer of Niobium|first = William P.|last = Griffith|author2=Morris, Peter J. T. |journal = Notes and Records of the Royal Society of London|volume = 57|issue = 3|pages = 299–316|date = 2003|jstor = 3557720|doi = 10.1098/rsnr.2003.0216}}</ref> এবং ১৮০৯ সালে ইংরেজ রসায়নবিদ [[উইলিয়াম হাইড ওল্লাস্টন]] এর অক্সাইড [[কলাম্বাইট|কলাম্বাইটের]] সাথে তুলনা করেছিলেন, with a density of 5.918 g/cm<sup>3</sup>, to that of tantalum, [[tantalite]] with a density of 7.935 g/cm<sup>3</sup>. He concluded that the two oxides, despite their difference in measured density, were identical and kept the name tantalum.<ref name="Wolla">{{cite journal|title = On the Identity of Columbium and Tantalum|pages = 246–252|journal = Philosophical Transactions of the Royal Society of London|first = William Hyde|last = Wollaston|authorlink = William Hyde Wollaston|doi = 10.1098/rstl.1809.0017| jstor = 107264|volume = 99|date = 1809}}</ref> After [[Friedrich Wöhler]] confirmed these results, it was thought that columbium and tantalum were the same element. This conclusion was disputed in 1846 by the German chemist [[Heinrich Rose]], who argued that there were two additional elements in the tantalite sample, and he named them after the children of [[Tantalus]]: niobium (from [[Niobe]], the goddess of tears), and pelopium (from [[Pelops]]).<ref name="Pelop">{{cite journal|title = Ueber die Zusammensetzung der Tantalite und ein im Tantalite von Baiern enthaltenes neues Metall|pages = 317–341|journal = Annalen der Physik|authorlink = Heinrich Rose|language=German|first = Heinrich|last = Rose|doi = 10.1002/andp.18441391006|url = http://gallica.bnf.fr/ark:/12148/bpt6k15148n/f327.table|volume = 139|issue = 10|date = 1844|bibcode = 1844AnP...139..317R }}</ref><ref>{{cite journal|title = Ueber die Säure im Columbit von Nordamérika|language=German|pages = 572–577|first = Heinrich|last = Rose|journal = Annalen der Physik|doi = 10.1002/andp.18471460410|url = http://gallica.bnf.fr/ark:/12148/bpt6k15155x/f586.table |date=1847| volume = 146|issue = 4|authorlink = Heinrich Rose|bibcode = 1847AnP...146..572R }}</ref> The supposed element "pelopium" was later identified as a mixture of tantalum and niobium, and it was found that the niobium was identical to the columbium already discovered in 1801 by Hatchett. |
|||
The differences between tantalum and niobium were demonstrated unequivocally in 1864 by [[Christian Wilhelm Blomstrand]],<ref name="Ilmen" /> and [[Henri Etienne Sainte-Claire Deville]], as well as by [[Louis J. Troost]], who determined the empirical formulas of some of their compounds in 1865.<ref name="Ilmen">{{cite journal|title = Tantalsäure, Niobsäure, (Ilmensäure) und Titansäure|journal = Fresenius' Journal of Analytical Chemistry|volume = 5|issue = 1|date = 1866|doi = 10.1007/BF01302537|pages = 384–389|author= Marignac, Blomstrand|author2= H. Deville|author3= L. Troost|author4= R. Hermann|last-author-amp= yes}}</ref><ref name="Gupta"/> Further confirmation came from the Swiss chemist [[Jean Charles Galissard de Marignac]],<ref>{{cite journal|journal = Annales de Chimie et de Physique|title = Recherches sur les combinaisons du niobium|pages = 7–75|authorlink = Jean Charles Galissard de Marignac|language=French| first = M. C.|last= Marignac|url = http://gallica.bnf.fr/ark:/12148/bpt6k34818t/f4.table|date= 1866|volume = 4|issue = 8}}</ref> in 1866, who proved that there were only two elements. These discoveries did not stop scientists from publishing articles about the so-called ''[[ilmenium]]'' until 1871.<ref>{{cite journal|title = Fortgesetzte Untersuchungen über die Verbindungen von Ilmenium und Niobium, sowie über die Zusammensetzung der Niobmineralien (Further research about the compounds of ilmenium and niobium, as well as the composition of niobium minerals)|first = R.|last = Hermann|journal = Journal für Praktische Chemie|language=German|volume = 3|issue = 1|pages =373–427|doi = 10.1002/prac.18710030137|date = 1871|url = https://zenodo.org/record/1427850}}</ref> De Marignac was the first to produce the metallic form of tantalum in 1864, when he [[redox|reduced]] tantalum chloride by heating it in an atmosphere of [[hydrogen]].<ref name="nauti">{{cite web|url = http://nautilus.fis.uc.pt/st2.5/scenes-e/elem/e04100.html|title = Niobium|publisher = Universidade de Coimbra|accessdate = 2008-09-05}}</ref> Early investigators had only been able to produce impure tantalum, and the first relatively pure ductile metal was produced by [[Werner von Bolton]] in [[Charlottenburg]] in 1903. Wires made with metallic tantalum were used for [[light bulb]] filaments until [[tungsten]] replaced it in widespread use.<ref>{{cite journal|title = Scanning Our Past from London The Filament Lamp and New Materials|journal = Proceedings of the IEEE|volume = 89|issue = 3|date = 2001|doi = 10.1109/5.915382|author = Bowers, B.|page = 413}}</ref> |
|||
{{রসায়ন-অসম্পূর্ণ}} |
|||
The name tantalum was derived from the name of the mythological [[Tantalus]], the father of [[Niobe]] in [[Greek mythology]]. In the story, he had been punished after death by being condemned to stand knee-deep in water with perfect fruit growing above his head, both of which eternally ''tantalized'' him. (If he bent to drink the water, it drained below the level he could reach, and if he reached for the fruit, the branches moved out of his grasp.)<ref>{{cite journal|journal = Journal of Social Sciences|volume = 1|issue = 4|pages = 238–239|date = 2005|first = Sule|last = Aycan|title = Chemistry Education and Mythology|doi = 10.3844/jssp.2005.238.239|url = http://thescipub.com/PDF/jssp.2005.238.239.pdf}}</ref> Anders Ekeberg wrote "This metal I call ''tantalum'' ... partly in allusion to its incapacity, when immersed in acid, to absorb any and be saturated."<ref>{{Greenwood&Earnshaw|page=1138}}</ref> |
|||
For decades, the commercial technology for separating tantalum from niobium involved the [[fractional crystallization (chemistry)|fractional crystallization]] of [[potassium heptafluorotantalate]] away from potassium oxypentafluoroniobate monohydrate, a process that was discovered by [[Jean Charles Galissard de Marignac]] in 1866. This method has been supplanted by [[solvent extraction]] from fluoride-containing solutions of tantalum.<ref name="Gupta">{{cite book|title = Extractive Metallurgy of Niobium|first = C. K.|last = Gupta|author2=Suri, A. K. |publisher = CRC Press|date = 1994|isbn = 978-0-8493-6071-8}}</ref> |
|||
==Characteristics== |
|||
===Physical properties=== |
|||
Tantalum is dark (blue-gray),<ref>{{cite book | chapter = Tantalum | chapter-url = https://books.google.com/?id=5o3Lr2Swz8sC&pg=PA204 | isbn = 978-0-86516-573-1 | title = Classical Mythology & More: A Reader Workbook | author1 = Colakis, Marianthe | author2 = Masello, Mary Joan | date = 2007-06-30}}</ref> dense, ductile, very hard, easily fabricated, and highly conductive of heat and electricity. The metal is renowned for its resistance to [[corrosion]] by [[acid]]s; in fact, at temperatures below 150 °[[Celsius|C]] tantalum is almost completely immune to attack by the normally aggressive [[aqua regia]]. It can be dissolved with [[hydrofluoric acid]] or acidic solutions containing the [[fluoride]] ion and [[sulfur trioxide]], as well as with a solution of [[potassium hydroxide]]. Tantalum's high melting point of 3017 °C (boiling point 5458 °C) is exceeded among the elements only by [[tungsten]], [[rhenium]] and [[osmium]] for metals, and [[carbon]]. |
|||
Tantalum exists in two crystalline phases, alpha and beta. The alpha phase is relatively [[Ductility|ductile]] and soft; it has [[body-centered cubic]] structure ([[space group]] ''Im3m'', lattice constant ''a'' = 0.33058 nm), [[Knoop hardness test|Knoop hardness]] 200–400 HN and electrical resistivity 15–60 µΩ⋅cm. The beta phase is hard and brittle; its crystal symmetry is [[tetragonal]] (space group ''P42/mnm'', ''a'' = 1.0194 nm, ''c'' = 0.5313 nm), Knoop hardness is 1000–1300 HN and electrical resistivity is relatively high at 170–210 µΩ⋅cm. The beta phase is metastable and converts to the alpha phase upon heating to 750–775 °C. Bulk tantalum is almost entirely alpha phase, and the beta phase usually exists as thin films<ref>{{cite journal|title=Electronic structure of β-Ta films from X-ray photoelectron spectroscopy and first-principles calculations|date=2019|last1=Magnuson|first1=M.|journal=Applied Surface Science|volume=470|page=607–612|last2=Greczynski|first2=G.|last3=Eriksson|first3=F.|last4=Hultman|first4=L.|last5=Hogberg|first5=H.|doi=10.1016/j.apsusc.2018.11.096|url=http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-152876}}</ref> obtained by magnetron |
|||
[[sputtering]], [[chemical vapor deposition]] or [[Electrochemistry|electrochemical deposition]] from an [[Eutectic system|eutectic]] molten salt solution.<ref>{{cite journal|doi=10.1016/j.surfcoat.2003.06.008|title=Texture, structure and phase transformation in sputter beta tantalum coating|date=2004|last1=Lee|first1=S.|journal=Surface and Coatings Technology|volume=177–178|page=44|last2=Doxbeck|first2=M.|last3=Mueller|first3=J.|last4=Cipollo|first4=M.|last5=Cote|first5=P.|url=https://zenodo.org/record/1259369}}</ref> |
|||
===Isotopes=== |
|||
{{Main|Isotopes of tantalum}} |
|||
Natural tantalum consists of two [[isotope]]s: <sup>180m</sup>Ta (0.012%) and <sup>181</sup>Ta (99.988%). <sup>181</sup>Ta is a [[stable isotope]]. <sup>180m</sup>Ta (''m'' denotes a metastable state) is predicted to decay in three ways: [[isomeric transition]] to the [[ground state]] of <sup>180</sup>Ta, [[beta decay]] to <sup>180</sup>[[Tungsten|W]], or electron capture to <sup>180</sup>[[Hafnium|Hf]]. However, radioactivity of this [[nuclear isomer]] has never been observed, and only a lower limit on its [[half-life]] of 2.0 × 10<sup>16</sup> years has been set.<ref>{{cite journal|last1=Hult|first1=Mikael|last2=Wieslander|first2=J. S. Elisabeth|last3=Marissens|first3=Gerd|last4=Gasparro|first4=Joël|last5=Wätjen|first5=Uwe|last6=Misiaszek|first6=Marcin|title=Search for the radioactivity of 180mTa using an underground HPGe sandwich spectrometer|doi=10.1016/j.apradiso.2009.01.057|pmid=19246206|volume=67|issue=5|journal=Applied Radiation and Isotopes|pages=918–921|year=2009}}</ref> The ground state of <sup>180</sup>Ta has a half-life of only 8 hours. <sup>180m</sup>Ta is the only naturally occurring [[nuclear isomer]] (excluding [[radiogenic]] and [[cosmogenic]] short-lived nuclides). It is also the rarest primordial isotope in the Universe, taking into account the elemental abundance of tantalum and isotopic abundance of <sup>180m</sup>Ta in the natural mixture of isotopes (and again excluding radiogenic and cosmogenic short-lived nuclides).<ref name="NUBASE">{{NUBASE 2003}}</ref> |
|||
Tantalum has been examined theoretically as a "[[Salted bomb|salting]]" material for [[nuclear weapon]]s ([[cobalt]] is the better-known hypothetical salting material). An external shell of <sup>181</sup>Ta would be irradiated by the intensive high-energy neutron flux from a hypothetical exploding nuclear weapon. This would transmute the tantalum into the radioactive isotope <sup>182</sup>Ta, which has a [[half-life]] of 114.4 days and produces [[gamma ray]]s with approximately 1.12 million electron-volts (MeV) of energy apiece, which would significantly increase the radioactivity of the [[nuclear fallout]] from the explosion for several months. Such "salted" weapons have never been built or tested, as far as is publicly known, and certainly never used as weapons.<ref>{{cite journal|last1=Win|first1=David Tin|last2=Al Masum|first2=Mohammed|title=Weapons of Mass Destruction|date=2003|journal=Assumption University Journal of Technology|volume=6|issue=4|pages=199–219|url=http://www.journal.au.edu/au_techno/2003/apr2003/aujt6-4_article07.pdf}}</ref> |
|||
Tantalum can be used as a target material for accelerated proton beams for the production of various short-lived isotopes including <sup>8</sup>Li, <sup>80</sup>Rb, and <sup>160</sup>Yb.<ref>{{cite web|url=https://mis.triumf.ca/science/planning/yield/target/Ta|title=Tantalum Target Yields - ISAC Yield Database - TRIUMF : Canada's National Laboratory for Particle and Nuclear Physics|website=mis.triumf.ca}}</ref> |
|||
==Chemical compounds== |
|||
Tantalum forms compounds in oxidation states −III to +V. Most commonly encountered are oxides of Ta(V), which includes all minerals. The chemical properties of Ta and Nb are very similar. In aqueous media, Ta only exhibit the +V oxidation state. Like niobium, tantalum is barely soluble in dilute solutions of [[Hydrochloric acid|hydrochloric]], [[Sulfuric acid|sulfuric]], [[Nitric acid|nitric]] and [[Phosphoric acid|phosphoric acids]] due to the precipitation of hydrous Ta(V) oxide.<ref name="Aguly" /> In basic media, Ta can be solubilized due to the formation of polyoxotantalate species.<ref>{{Cite journal|last=Deblonde|first=Gauthier J. -P.|last2=Chagnes|first2=Alexandre|last3=Bélair|first3=Sarah|last4=Cote|first4=Gérard|date=2015-07-01|title=Solubility of niobium(V) and tantalum(V) under mild alkaline conditions|journal=Hydrometallurgy|volume=156|pages=99–106|doi=10.1016/j.hydromet.2015.05.015|issn=0304-386X}}</ref> |
|||
===Oxides, nitrides, carbides, sulfides=== |
|||
[[Tantalum pentoxide]] (Ta<sub>2</sub>O<sub>5</sub>) is the most important compound from the perspective of applications. Oxides of tantalum in lower oxidation states are numerous, including many [[defect structure]]s, and are lightly studied or poorly characterized.<ref>{{Greenwood&Earnshaw2nd}}</ref> |
|||
Tantalates, compounds containing [TaO<sub>4</sub>]<sup>3−</sup> or [TaO<sub>3</sub>]<sup>−</sup> are numerous. [[Lithium tantalate]] (LiTaO<sub>3</sub>) adopts a perovskite structure. [[Lanthanum]] tantalate (LaTaO<sub>4</sub>) contains isolated {{chem|TaO|4|3−}} tetrahedra.<ref name="HollemanAF">{{cite book|title=Lehrbuch der Anorganischen Chemie|date=2007|publisher=de Gruyter|isbn=978-3-11-017770-1|edition=102nd|location=|pages=|language=German|author=Holleman, A. F.|author2=Wiberg, E.|author3=Wiberg, N.}}</ref> |
|||
As in the cases of other [[refractory metal]]s, the hardest known compounds of tantalum are nitrides and carbides. [[Tantalum carbide]], TaC, like the more commonly used [[tungsten carbide]], is a hard [[ceramic]] that is used in cutting tools. Tantalum(III) nitride is used as a thin film insulator in some microelectronic fabrication processes.<ref>{{cite journal|title=Microstructure of amorphous tantalum nitride thin films|first=S.|last=Tsukimoto| author2= Moriyama, M.| author3= Murakami, Masanori| journal=Thin Solid Films|date=1961|volume= 460|issue=1–2|pages=222–226|doi=10.1016/j.tsf.2004.01.073|bibcode = 2004TSF...460..222T }}</ref> |
|||
The best studied chalcogenide is TaS<sub>2</sub>, a layered [[semiconductor]], as seen for other [[transition metal dichalcogenide]]s. A tantalum-tellurium alloy forms [[quasicrystal]]s.<ref name="HollemanAF"/> |
|||
===Halides=== |
|||
Tantalum halides span the oxidation states of +5, +4, and +3. Tantalum pentafluoride (TaF<sub>5</sub>) is a white solid with a melting point of 97.0 °C. The anion [TaF<sub>7</sub>]<sup>2-</sup> is used for its separation from niobium.<ref name="ICE">{{cite journal|title=Staff-Industry Collaborative Report: Tantalum and Niobium|first=Donald J.|last=Soisson|author2=McLafferty, J. J. |author3=Pierret, James A. | journal=Ind. Eng. Chem.|date=1961|volume= 53|issue=11|pages=861–868|doi=10.1021/ie50623a016}}</ref> The chloride {{chem|TaCl|5}}, which exists as a dimer, is the main reagent in synthesis of new Ta compounds. It hydrolyzes readily to an [[oxychloride]]. The lower halides {{chem|TaX|4}} and {{chem|TaX|3}}, feature Ta-Ta bonds.<ref name="HollemanAF"/><ref name="Aguly">{{cite book|first=Anatoly|last=Agulyansky|title=The Chemistry of Tantalum and Niobium Fluoride Compounds|publisher=Elsevier|date=2004| isbn=978-0-444-51604-6| url=https://books.google.com/?id=Z-4QXNB5Hp8C|accessdate=2008-09-02}}</ref><ref name="HollemanAF"/> |
|||
===Organotantalum compounds=== |
|||
[[Organotantalum chemistry|Organotantalum compound]]s include [[pentamethyltantalum]], mixed alkyltantalum chlorides, alkyltantalum hydrides, alkylidene complexes as well as cyclopentadienyl derivatives of the same.<ref name=Schrock>{{Cite journal|last=Schrock|first=Richard R.|date=1979-03-01|title=Alkylidene complexes of niobium and tantalum|journal=Accounts of Chemical Research|volume=12|issue=3|pages=98–104|doi=10.1021/ar50135a004|issn=0001-4842}}</ref><ref>{{cite journal|doi=10.1021/om701189e|title=Ethylene Complexes of the Early Transition Metals: Crystal Structures of {{chem|[HfEt|4|(C|2|H|4|)|2-|]}} and the Negative-Oxidation-State Species {{chem|[TaHEt(C|2|H|4|)|3|3-|]}} and {{chem|[WH(C|2|H|4|)|4|3-|]}}|author=Morse, P. M.|journal=Organometallics|date=2008|volume=27|issue=5|page=984|displayauthors=1|author2=Shelby, Q. D. |author3=Kim, D. Y. |author4=Girolami, G. S. |last-author-amp=yes}}</ref> Diverse salts and substituted derivatives are known for the hexacarbonyl [Ta(CO)<sub>6</sub>]<sup>−</sup> and related [[isocyanide]]s. |
|||
[[File:DOSBIWoneRotamer.png|144px|thumb|Ta(CH<sub>3</sub>)<sub>5</sub>.]] |
|||
==Occurrence== |
|||
[[File:Tantalite.jpg|thumb|left|Tantalite, [[Pilbara|Pilbara district]], Australia]] |
|||
Tantalum is estimated to make up about 1 [[Parts per million|ppm]]<ref name="Emsley">{{cite book|title = Nature's Building Blocks: An A-Z Guide to the Elements|last = Emsley|first= John|publisher = Oxford University Press|date = 2001|location = Oxford, England, UK|isbn = 978-0-19-850340-8|chapter = Tantalum|page=420}}</ref> or 2 [[Parts per million|ppm]]<ref name="Aguly"/> of the [[Abundance of elements in Earth's crust|Earth's crust by weight]]. There are many species of tantalum minerals, only some of which are so far being used by industry as raw materials: [[tantalite]] (a series consisting of tantalite-(Fe), tantalite-(Mn) and tantalite-(Mg)) [[microlite]] (now a group name), [[wodginite]], [[euxenite]] (actually euxenite-(Y)), and [[polycrase]] (actually polycrase-(Y)).<ref name="mindat.org"/> Tantalite ([[iron|Fe]], [[manganese|Mn]])Ta<sub>2</sub>[[oxygen|O]]<sub>6</sub> is the most important mineral for tantalum extraction. Tantalite has the same mineral structure as [[columbite]] ([[iron|Fe]], [[manganese|Mn]]) (Ta, [[niobium|Nb]])<sub>2</sub>[[oxygen|O]]<sub>6</sub>; when there is more tantalum than niobium it is called tantalite and when there is more niobium than tantalum is it called columbite (or [[niobite]]). The high density of tantalite and other tantalum containing minerals makes the use of [[Gravity separation|gravitational separation]] the best method. Other minerals include [[samarskite]] and [[fergusonite]]. |
|||
[[File:World Tantalum Production 2015.svg|upright=1.4|thumb|Tantalum producers in 2015 with Rwanda being the main producer|alt=Grey and white world map with China, Australia, Brazil and Kongo colored blue representing less than 10% of the tantalum world production each and Rwanda colored in green representing 60% of tantalum world production]] |
|||
The primary mining of tantalum is in [[Australia]], where the largest producer, [[Global Advanced Metals]], formerly known as [[Talison Minerals]], operates two mines in Western Australia, [[Greenbushes, Western Australia|Greenbushes]] in the Southwest and [[Wodgina mine|Wodgina]] in the [[Pilbara]] region. The Wodgina mine was reopened in January 2011 after mining at the site was suspended in late-2008 due to the global financial crisis.<ref>{{cite news| url = https://af.reuters.com/article/drcNews/idAFLDE6530TW20100609 | work = Reuters |title = Talison Tantalum eyes mid-2011 Wodgina restart 2010-06-09 | accessdate = 2010-08-27 | date=2010-06-09}}</ref> Less than a year after it reopened, Global Advanced Metals announced that due to again "... softening tantalum demand ...", and other factors, tantalum mining operations were to cease at the end of February 2012.<ref name="Wodgina-tant-closed">{{cite news| url=http://au.news.yahoo.com/thewest/business/a/-/business/12702333/gam-closes-wodgina-tantalum-mine/| title=GAM closes Wodgina tantalum mine| last=Emery| first=Kate| date=24 Jan 2012| work=[[The West Australian]]| accessdate=20 March 2012| quote=Worldwide softening tantalum demand and delays in receiving Governmental approval for installation of necessary crushing equipment are among contributing factors in this decision| url-status=dead| archiveurl=https://web.archive.org/web/20121204055041/http://au.news.yahoo.com/thewest/business/a/-/business/12702333/gam-closes-wodgina-tantalum-mine/| archivedate=4 December 2012}} |
|||
</ref> Wodgina produces a primary tantalum concentrate which is further upgraded at the Greenbushes operation before being sold to customers.<ref name="Talison">{{cite web|publisher = Global Advanced Metals |date = 2008|url = http://globaladvancedmetals.com/our-operations/gam-resources/wodgina-australia.aspx |title = Wodgina Operations|accessdate = 2011-03-28}}</ref> Whereas the large-scale producers of niobium are in [[Brazil]] and [[Canada]], the ore there also yields a small percentage of tantalum. Some other countries such as [[China]], [[Ethiopia]], and [[Mozambique]] mine ores with a higher percentage of tantalum, and they produce a significant percentage of the world's output of it. Tantalum is also produced in [[Thailand]] and [[Malaysia]] as a by-product of the [[tin]] mining there. During gravitational separation of the ores from placer deposits, not only is [[cassiterite]] (SnO<sub>2</sub>) found, but a small percentage of tantalite also included. The slag from the tin smelters then contains economically useful amounts of tantalum, which is leached from the slag.<ref name="Gupta"/><ref name="USGS2006">{{cite web|publisher = US Geological Survey|last = Papp|first = John F.|title = 2006 Minerals Yearbook Nb & Ta|date = 2006|url = http://minerals.usgs.gov/minerals/pubs/commodity/niobium/#pubs|accessdate = 2008-06-03}}</ref> |
|||
[[File:World Tantalum Production 2006.svg|upright=1.4|thumb|Tantalum producers in 2006 with Australia being the main producer|alt=Grey and white world map with Canada, Brazil and Mozambique colored blue representing less than 20% of the tantalum world production each and Australia colored in green representing 60% of tantalum world production]] |
|||
World tantalum mine production has undergone an important geographic shift since the start of the 21st century when production was predominantly from Australia and Brazil. Beginning in 2007 and through 2014, the major sources of tantalum production from mines dramatically shifted to the DRC, Rwanda, and some other African countries.<ref>{{cite web| url = http://pubs.usgs.gov/fs/2015/3079/fs20153079.pdf | title = Shift in Global Tantalum Mine Production, 2000–2014 | last1 = Bleiwas | first1 = Donald I. | last2 =Papp| first2 = John F.| last3 =Yager | first3 = Thomas R. | publisher = U.S. Geological Survey | year = 2015}}</ref> Future sources of supply of tantalum, in order of estimated size, are being explored in [[Saudi Arabia]], [[Egypt]], [[Greenland]], [[China]], [[Mozambique]], [[Canada]], [[Australia]], the [[United States]], [[Finland]], and [[Brazil]].<ref name="Mining Journal">{{cite journal|journal = Mining Journal|author = M. J.|title = Tantalum supplement|date = November 2007|url = http://www.noventa.net/pdf/presentations/tanatalumSCR_presentation.pdf|accessdate = 2008-06-03}}</ref><ref>{{cite journal|url = http://www.doir.wa.gov.au/documents/gswa/gsdMRB_22_chap10.pdf|archiveurl = https://web.archive.org/web/20070926195547/http://www.doir.wa.gov.au/documents/gswa/gsdMRB_22_chap10.pdf|archivedate = 2007-09-26|journal = GSWA Mineral Resources Bulletin|volume = 22|title = International tantalum resources — exploration and mining|issue = 10}}</ref> |
|||
It is estimated that there are less than 50 years left of tantalum resources, based on extraction at current rates, demonstrating the need for increased [[recycling]].<ref>{{cite web|url = http://www.scientificamerican.com/article.cfm?id=how-much-is-left |title=How much is left?|accessdate=2013-01-13}}</ref> |
|||
==Status as a conflict resource== |
|||
{{See also|Coltan mining and ethics|Coltan#Ethics of mining in the Democratic Republic of Congo}} |
|||
Tantalum is considered a [[conflict resource]]. [[Coltan]], the industrial name for a [[columbite]]–[[tantalite]] mineral from which niobium and tantalum are extracted,<ref>[http://www.tanb.org/coltan Tantalum-Niobium International Study Center: Coltan] Retrieved 2008-01-27</ref> can also be found in [[Central Africa]], which is why tantalum is being linked to [[Second Congo War|warfare in the Democratic Republic of the Congo]] (formerly [[Zaire]]). According to an October 23, 2003 [[United Nations]] report,<ref>{{cite web|title = S/2003/1027|date = 2003-10-26|url = https://www.un.org/Docs/journal/asp/ws.asp?m=S/2003/1027|accessdate =2008-04-19}}</ref> the smuggling and exportation of coltan has helped fuel the war in the Congo, a crisis that has resulted in approximately 5.4 million deaths since 1998<ref>{{cite web|publisher = International Rescue Committee|title = Special Report: Congo|url = http://www.rescue.org/special-reports/special-report-congo-y|accessdate = 2008-04-19}}</ref> – making it the world’s deadliest documented conflict since [[World War II]]. Ethical questions have been raised about responsible corporate behavior, human rights, and endangering wildlife, due to the exploitation of resources such as coltan in the armed conflict regions of the Congo Basin.<ref>{{cite book|title = Coltan Mining in the Democratic Republic of Congo: How tantalum-using industries can commit to the reconstruction of the DRC|first = Karen|last = Hayes|author2=Burge, Richard |journal = Fauna & Flora|isbn = 978-1-903703-10-6|pages = 1–64|year = 2003}}</ref><ref>{{cite web|url = http://pulitzercenter.org/video/congos-bloody-coltan|date=January 6, 2011|work=Pulitzer Center on Crisis Reporting|author=Dizolele, Mvemba Phezo|title=Congo's Bloody Coltan|accessdate=2009-08-08}}</ref><ref>{{cite web|url=http://www1.american.edu/ted/ice/congo-coltan.htm|title=Congo War and the Role of Coltan|accessdate=2009-08-08|url-status=dead|archiveurl=https://web.archive.org/web/20090713173610/http://www1.american.edu/TED/ice/congo-coltan.htm|archivedate=2009-07-13}}</ref><ref>{{cite web|url = http://www.panda.org/what_we_do/where_we_work/congo_basin_forests/problems/mining/coltan_mining/ |archiveurl = https://web.archive.org/web/20090330005811/http://www.panda.org/what_we_do/where_we_work/congo_basin_forests/problems/mining/coltan_mining/ |archivedate = 2009-03-30 |title=Coltan mining in the Congo River Basin|accessdate =2009-08-08}}</ref> However, although important for the local economy in Congo, the contribution of coltan mining in Congo to the world supply of tantalum is usually small. The [[United States Geological Survey]] reports in its yearbook that this region produced a little less than 1% of the world's tantalum output in 2002–2006, peaking at 10% in 2000 and 2008.<ref name="USGS2006"/> |
|||
The stated aim of the ''Solutions for Hope Tantalum Project'' is to "source conflict-free tantalum from the Democratic Republic of Congo"<ref>{{cite web|title='Solutions for Hope' Tantalum Project Offers Solutions and Brings Hope to the People of the DRC|url=http://solutions-network.org/site-sfhtantalum/|website=Solutions Network|accessdate=18 September 2014}}</ref> |
|||
== Production and fabrication == |
|||
[[File:Tantalum world production.svg|thumb|upright=1|Time trend of tantalum production until 2012<ref>{{Cite web|url=http://minerals.usgs.gov/ds/2005/140|archiveurl=https://web.archive.org/web/20130604121254/http://minerals.usgs.gov/ds/2005/140/|url-status=dead|title=Mineral Resources Program|archivedate=June 4, 2013|website=minerals.usgs.gov}}</ref>]] |
|||
Several steps are involved in the extraction of tantalum from tantalite. First, the mineral is [[crusher|crushed]] and concentrated by [[gravity separation]]. This is generally carried out near the [[mining|mine]] site. |
|||
=== Refining === |
|||
The refining of tantalum from its ores is one of the more demanding separation processes in industrial metallurgy. The chief problem is that tantalum ores contain significant amounts of [[niobium]], which has chemical properties almost identical to those of Ta. A large number of procedures have been developed to address this challenge. |
|||
In modern times, the separation is achieved by [[hydrometallurgy]].<ref name=Chang>{{cite journal|title=Solvent extraction technology for the separation and purification of niobium and tantalum: A review|author1=Zhaowu Zhu|author2=Chu Yong Cheng|journal=Hydrometallurgy|volume=107|issue=1–2|year=2011|pages=1–12|doi=10.1016/j.hydromet.2010.12.015}}</ref> Extraction begins with [[Leaching (metallurgy)|leaching]] the ore with [[hydrofluoric acid]] together with [[sulfuric acid]] or [[hydrochloric acid]]. This step allows the tantalum and niobium to be separated from the various non-metallic impurities in the rock. Although Ta occurs as various minerals, it is conveniently represented as the pentoxide, since most oxides of tantalum(V) behave similarly under these conditions. A simplified equation for its extraction is thus: |
|||
: Ta<sub>2</sub>O<sub>5</sub> + 14 HF → 2 H<sub>2</sub>[TaF<sub>7</sub>] + 5 H<sub>2</sub>O |
|||
Completely analogous reactions occur for the niobium component, but the hexafluoride is typically predominant under the conditions of the extraction. |
|||
: Nb<sub>2</sub>O<sub>5</sub> + 12 HF → 2 H[NbF<sub>6</sub>] + 5 H<sub>2</sub>O |
|||
These equations are simplified: it is suspected that bisulfate (HSO<sub>4</sub><sup>−</sup>) and chloride compete as ligands for the Nb(V) and Ta(V) ions, when sulfuric and hydrochloric acids are used, respectively.<ref name=Chang/> The tantalum and niobium fluoride complexes are then removed from the [[aqueous]] solution by [[Liquid–liquid extraction|liquid-liquid extraction]] into [[organic solvents]], such as [[cyclohexanone]], [[octanol]], and [[methyl isobutyl ketone]]. This simple procedure allows the removal of most metal-containing impurities (e.g. iron, manganese, titanium, zirconium), which remain in the aqueous phase in the form of their [[fluoride]]s and other complexes. |
|||
Separation of the tantalum ''from'' niobium is then achieved by lowering the [[ionic strength]] of the acid mixture, which causes the niobium to dissolve in the aqueous phase. It is proposed that [[oxyfluoride]] H<sub>2</sub>[NbOF<sub>5</sub>] is formed under these conditions. Subsequent to removal of the niobium, the solution of purified H<sub>2</sub>[TaF<sub>7</sub>] is neutralised with aqueous [[ammonia]] to precipitate hydrated tantalum oxide as a solid, which can be [[calcination|calcined]] to [[tantalum pentoxide]] (Ta<sub>2</sub>O<sub>5</sub>).<ref>{{cite book|last=Agulyanski|first=Anatoly|title=Chemistry of Tantalum and Niobium Fluoride Compounds|date=2004|publisher=Elsevier|location=Burlington|isbn=9780080529028|edition=1st}}</ref> |
|||
Instead of hydrolysis, the H<sub>2</sub>[TaF<sub>7</sub>] can be treated with [[potassium fluoride]] to produce [[potassium heptafluorotantalate]]: |
|||
: H<sub>2</sub>[TaF<sub>7</sub>] + 2 KF → K<sub>2</sub>[TaF<sub>7</sub>] + 2 HF |
|||
Unlike H<sub>2</sub>[TaF<sub>7</sub>], the potassium salt is readily crystallized and handled as a solid. |
|||
K<sub>2</sub>[TaF<sub>7</sub>] can be converted to metallic tantalum by [[reduction-oxidation|reduction]] with [[sodium]], at approximately 800 °C in [[molten salt]].<ref>{{cite journal|last=Okabe|first=Toru H.|author2=Sadoway, Donald R. |title=Metallothermic reduction as an electronically mediated reaction|journal=Journal of Materials Research|date=1998|volume=13|issue=12|pages=3372–3377|doi=10.1557/JMR.1998.0459|bibcode = 1998JMatR..13.3372O }}</ref> |
|||
: K<sub>2</sub>[TaF<sub>7</sub>] + 5 Na → Ta + 5 NaF + 2 KF |
|||
In an older method, called the [[Jean Charles Galissard de Marignac|Marignac]] process, the mixture of H<sub>2</sub>[TaF<sub>7</sub>] and H<sub>2</sub>[NbOF<sub>5</sub>] was converted to a ''mixture'' of K<sub>2</sub>[TaF<sub>7</sub>] and K<sub>2</sub>[NbOF<sub>5</sub>], which was then be separated by [[Fractional crystallization (chemistry)|fractional crystallization]], exploiting their different water solubilities. |
|||
=== Electrolysis === |
|||
{{See also|FFC Cambridge process}} |
|||
Tantalum can also be refined by electrolysis, using a modified version of the [[Hall–Héroult process]]. Instead of requiring the input oxide and output metal to be in liquid form, tantalum electrolysis operates on non-liquid powdered oxides. The initial discovery came in 1997 when Cambridge University researchers immersed small samples of certain oxides in baths of molten salt and reduced the oxide with electric current. The cathode uses powdered metal oxide. The anode is made of carbon. The molten salt at {{convert|1000|C|F}} is the electrolyte. The first refinery has enough capacity to supply 3–4% of annual global demand.<ref>{{cite news|url=https://www.economist.com/news/science-and-technology/21571847-exotic-useful-metals-such-tantalum-and-titanium-are-about-become-cheap |title=Manufacturing metals: A tantalising prospect |newspaper=The Economist |date=2013-02-16 |accessdate=2013-04-17}}</ref> |
|||
=== Fabrication and metalworking === |
|||
All [[welding]] of tantalum must be done in an inert atmosphere of [[argon]] or [[helium]] in order to shield it from contamination with atmospheric gases. Tantalum is not [[solderable]]. Grinding tantalum is difficult, especially so for [[Annealing (metallurgy)|annealed]] tantalum. In the annealed condition, tantalum is extremely [[ductile]] and can be readily formed as metal sheets.<ref>{{Cite web|url = http://www.nfpa.org/assets/files/aboutthecodes/484/nfpa484-2002.pdf|title = NFPA 484 – Standard for Combustible Metals, Metal Powders, and Metal Dusts – 2002 Edition|date = 2002-08-13|access-date = 2016-02-12|website = National Fire Protection Association|publisher = NFPA|last = |first = }}</ref> |
|||
==Applications== |
|||
===Electronics=== |
|||
[[File:Tantal-Perle-Wiki-07-02-25-P1040364b.jpg|thumb|upright|Tantalum electrolytic capacitor]] |
|||
The major use for tantalum, as the metal powder, is in the production of electronic components, mainly [[capacitor]]s and some high-power [[resistor]]s. [[tantalum capacitor|Tantalum electrolytic capacitors]] exploit the tendency of tantalum to form a protective [[oxide]] surface layer, using tantalum powder, pressed into a pellet shape, as one "plate" of the capacitor, the oxide as the [[dielectric]], and an electrolytic solution or conductive solid as the other "plate". Because the [[Relative static permittivity|dielectric layer]] can be very thin (thinner than the similar layer in, for instance, an aluminium electrolytic capacitor), a high [[capacitance]] can be achieved in a small volume. Because of the size and weight advantages, tantalum capacitors are attractive for [[portable telephone]]s, [[personal computer]]s, [[automotive electronics]] and [[cameras]].<ref name="USGSCR08">{{cite web|url = http://minerals.usgs.gov/minerals/pubs/commodity/niobium/mcs-2008-tanta.pdf|title = Commodity Report 2008: Tantalum|publisher = United States Geological Survey|accessdate = 2008-10-24}}</ref> |
|||
===Alloys=== |
|||
Tantalum is also used to produce a variety of [[alloy]]s that have high melting points, strength, and ductility. Alloyed with other metals, it is also used in making carbide tools for metalworking equipment and in the production of [[superalloy]]s for jet engine components, chemical process equipment, [[nuclear reactor]]s, missile parts, heat exchangers, tanks, and vessels.<ref>{{Cite news|url=https://www.admatinc.com/tantalum/sheetplate/|title=Tantalum Products: Tantalum Sheet & Plate {{!}} Admat Inc|work=Admat Inc.|access-date=2018-08-28|language=en-US}}</ref><ref name="USGSCR08"/><ref>{{cite journal|title = New applications for tantalum and tantalum alloys|journal = JOM Journal of the Minerals, Metals and Materials Society|volume = 52|issue = 3|date = 2000|doi = 10.1007/s11837-000-0100-6|page=40|first = R. W.|last = Buckman Jr.|bibcode = 2000JOM....52c..40B }}</ref> Because of its ductility, tantalum can be drawn into fine wires or filaments, which are used for evaporating metals such as [[aluminium]]. Since it resists attack by body fluids and is nonirritating, tantalum is widely used in making surgical instruments and implants. For example, porous tantalum coatings are used in the construction of orthopedic implants due to tantalum's ability to form a direct bond to hard tissue.<ref>{{cite journal|first = R.|last = Cohen|date = 2006|title = Applications of porous tantalum in total hip arthroplasty|journal = Journal of the American Academy of Orthopaedic Surgeons|volume = 14|pmid=17077337|last2 = Della Valle|first2 = C. J.|last3 = Jacobs|first3 = J. J.|issue = 12|pages = 646–55|doi = 10.5435/00124635-200611000-00008}}</ref> |
|||
Tantalum is inert against most acids except [[hydrofluoric acid]] and hot [[sulfuric acid]], and hot [[alkaline]] solutions also cause tantalum to corrode. This property makes it a useful metal for chemical reaction vessels and pipes for corrosive liquids. Heat exchanging coils for the steam heating of hydrochloric acid are made from tantalum.<ref name="Balke">{{cite journal|page= 1166|journal = Industrial and Engineering Chemistry|volume = 20|issue = 10|title = Columbium and Tantalum|first = Clarence W.|last = Balke|doi=10.1021/ie50310a022|date= 1935}}</ref> Tantalum was extensively used in the production of [[ultra high frequency]] [[Vacuum tube|electron tubes]] for radio transmitters. Tantalum is capable of capturing oxygen and nitrogen by forming nitrides and oxides and therefore helped to sustain the high vacuum needed for the tubes when used for internal parts such as grids and plates.<ref name="ICE"/><ref name="Balke"/> |
|||
===Other uses=== |
|||
[[File:Tantalio.png|thumb|320x320px|[[Bi-metallic coin|Bimetallic]] coins minted by the Bank of [[Kazakhstan]] with silver ring and tantalum center.]] |
|||
The high melting point and oxidation resistance lead to the use of the metal in the production of [[vacuum furnace]] parts. Tantalum is extremely inert and is therefore formed into a variety of corrosion resistant parts, such as [[thermowell]]s, valve bodies, and tantalum fasteners. Due to its high density, [[shaped charge]] and [[explosively formed penetrator]] liners have been constructed from tantalum.<ref>{{cite journal|title = Microstructure of high-strain, high-strain-rate deformed tantalum|first = Sia|last = Nemat-Nasser|author2 = Isaacs, Jon B.|author3 = Liu, Mingqi|journal = Acta Materialia|volume = 46|page= 1307|date = 1998|doi = 10.1016/S1359-6454(97)00746-5|issue = 4}}</ref> Tantalum greatly increases the armor penetration capabilities of a shaped charge due to its high density and high melting point.<ref>{{cite journal|doi = 10.1016/S0734-743X(01)00135-X|title = The penetration resistance of a titanium alloy against jets from tantalum shaped charge liners|date = 2001|last1 = Walters|first1 = William|author2 = Cooch, William|author3 = Burkins, Matthew|journal = International Journal of Impact Engineering|volume = 26|issue = 1–10|page= 823|last4 = Burkins|first4 = Matthew|url = https://zenodo.org/record/1260077}}</ref><ref>{{cite book|isbn = 978-0-471-64952-6|author = Russell, Alan M.|author2 = Lee, Kok Loong| date = 2005|publisher = Wiley-Interscience|location = Hoboken, NJ|title = Structure-property relations in nonferrous metals|url = https://books.google.com/?id=fIu58uZTE-gC&pg=PA129&lpg=PP128#PPA218|page = 218}}</ref> <!--http://www.meyersgroup.ucsd.edu/papers/journals/Meyers 176.pdf LE Murr, S Pappu, C Kennedy, CS Niou, M Meyers – Tantalum, 1996 http://www.osti.gov/bridge/servlets/purl/274177-TGUy10/webviewable/274177.pdf--> It is also occasionally used in precious [[watch]]es e.g. from [[Audemars Piguet]], [[F.P. Journe]], [[Hublot]], [[Montblanc (pens)|Montblanc]], [[Omega Watches|Omega]], and [[Panerai]]. Tantalum is also highly bioinert and is used as an orthopedic implant material.<ref name="Gerald L. Burke 1940">{{cite journal|journal = Canadian Medical Association Journal |author = Gerald L. Burke |date = 1940 |title =The Corrosion of Metals in Tissues; and An Introduction to Tantalum |volume = 43}}</ref> The high stiffness of tantalum makes it necessary to use it as highly porous foam or scaffold with lower stiffness for hip replacement implants to avoid [[stress shielding]].<ref>{{cite journal|journal = Clinical Materials|date = 1994|volume = 16|issue = 3|pages =167–173|title = Biological performance of tantalum|last = Black|first = J.|pmid=10172264|doi = 10.1016/0267-6605(94)90113-9}}</ref> Because tantalum is a non-ferrous, non-magnetic metal, these implants are considered to be acceptable for patients undergoing MRI procedures.<ref name="PaganiasTsakotos2012">{{cite journal|last1=Paganias|first1=Christos G.|last2=Tsakotos|first2=George A.|last3=Koutsostathis|first3=Stephanos D.|last4=Macheras|first4=George A.|title=Osseous integration in porous tantalum implants|journal=Indian Journal of Orthopaedics|volume=46|issue=5|year=2012|pages=505–13|issn=0019-5413|doi=10.4103/0019-5413.101032|pmid=23162141|pmc=3491782}}</ref><!--10.1016/j.actbio.2010.01.046 --> The oxide is used to make special high [[refractive index]] [[glass]] for [[camera]] lenses.<ref>{{cite book|title = Optical Materials: An Introduction to Selection and Application|chapter = Optical Glass Composition|first = Solomon|last = Musikant|publisher = CRC Press|date = 1985|page = 28|isbn = 978-0-8247-7309-0|chapter-url = https://books.google.com/?id=iJEXMF3JBtQC&pg=PA28}}</ref> |
|||
== Environmental issues == |
|||
Tantalum receives far less attention in the environmental field than it does in other geosciences. Upper Crust Concentrations (UCC) and the Nb/Ta ratio in the upper crust and in minerals are available because these measurements are useful as a geochemical tool.<ref>{{Cite journal|last=Green|first=TH.|date=1995|title=Significance of Nb/Ta as an indicator of geochemical processes in the crust-mantle system|journal=Chemical Geology|volume=120|issue=3–4|pages=347–359|bibcode=1995ChGeo.120..347G|doi=10.1016/0009-2541(94)00145-X}}</ref> The latest values for UCC and the Nb/Ta(w/w) ratio in the upper crust stand at 0.92 ppm and 12.7 respectively.<ref>{{Cite journal|last=Hu|first=Z.|last2=Gao|first2=S.|date=2008|title=Upper crustal abundances of trace elements: a revision and update|journal=Chemical Geology|volume=253|issue=3–4|pages=205|bibcode=2008ChGeo.253..205H|doi=10.1016/j.chemgeo.2008.05.010}}</ref> |
|||
Little data is available on tantalum concentrations in the different environmental compartments, especially in natural waters where reliable estimates of ‘dissolved’ tantalum concentrations in seawater and freshwaters have not even been produced.<ref name=":0">{{Cite journal|last=Filella|first=M.|date=2017|title=Tantalum in the environment|journal=Earth-Science Reviews|volume=173|pages=122–140|doi=10.1016/j.earscirev.2017.07.002}}</ref> Some values on dissolved concentrations in oceans have been published, but they are contradictory. Values in freshwaters fare little better, but, in all cases, they are probably below 1 ng L<sup>−1,</sup>since ‘dissolved’ concentrations in natural waters are well below most current analytical capabilities.<ref>{{Cite journal|last=Filella|first=M.|last2=Rodushkin|first2=I.|date=2018|title=A concise guide for the determination of less-studied technology-critical elements (Nb, Ta, Ga, In, Ge, Te) by inductively coupled plasma mass spectrometry in environmental samples|journal=Spectrochimica Acta Part B|volume=141|pages=80–84|doi=10.1016/j.sab.2018.01.004}}</ref> Analysis requires pre-concentration procedures that, for the moment, do not give consistent results. And in any case, tantalum appears to be present in natural waters mostly as particulate matter rather than dissolved.<ref name=":0" /> |
|||
Values for concentrations in soils, bed sediments and atmospheric aerosols are easier to come by.<ref name=":0" /> Values in soils are close to 1 ppm and thus to UCC values. This indicates detrital origin. For atmospheric aerosols the values available are scattered and limited. When tantalum enrichment is observed, it is probably due to loss of more water-soluble elements in aerosols in the clouds.<ref>{{Cite journal|last=Vlastelic|first=I.|last2=Suchorski|first2=K.|last3=Sellegri|first3=K.|last4=Colomb|first4=A.|last5=Nauret|first5=F.|last6=Bouvier|first6=L.|last7=Piro|first7=J-L.|date=2015|title=The high field strength element budget of atmospheric aerosols (puy de Dôme, France)|journal=Geochimica et Cosmochimica Acta|volume=167|pages=253–268|jstor=|bibcode=2015GeCoA.167..253V|doi=10.1016/j.gca.2015.07.006}}</ref> |
|||
Pollution linked to human use of the element has not been detected.<ref>{{Cite journal|last=Filella|first=M.|last2=Rodríguez-Murillo|first2=JC.|date=2017|title=Less-studied TCE: are their environmental concentrations increasing due to their use in new technologies?|journal=Chemosphere|volume=182|pages=605–616|doi=10.1016/j.chemosphere.2017.05.024|pmid=28525874}}</ref> Tantalum appears to be a very conservative element in biogeochemical terms, but its cycling and reactivity are still not fully understood. |
|||
==Precautions== |
|||
Compounds containing tantalum are rarely encountered in the laboratory. The metal is highly [[biocompatible]]<ref name="Gerald L. Burke 1940"/> and is used for body [[implant (medicine)|implants]] and [[coating]]s, therefore attention may be focused on other elements or the physical nature of the [[chemical compound]].<ref>{{cite journal|journal = Biomaterials|author = Matsuno H|author2 = Yokoyama A|author3 = Watari F|author4 = Uo M|author5 = Kawasaki T.|date = 2001|volume = 22|title = Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biocompatibility of tantalum.|doi = 10.1016/S0142-9612(00)00275-1|pmid=11336297|issue = 11|pages = 1253–62}}</ref> |
|||
People can be exposed to tantalum in the workplace by breathing it in, skin contact, or eye contact. The [[Occupational Safety and Health Administration]] (OSHA) has set the legal limit ([[permissible exposure limit]]) for tantalum exposure in the workplace as 5 mg/m<sup>3</sup> over an 8-hour workday. The [[National Institute for Occupational Safety and Health]] (NIOSH) has set a [[recommended exposure limit]] (REL) of 5 mg/m<sup>3</sup> over an 8-hour workday and a short-term limit of 10 mg/m<sup>3</sup>. At levels of 2500 mg/m<sup>3</sup>, tantalum is [[IDLH|immediately dangerous to life and health]].<ref>{{Cite web|title = CDC – NIOSH Pocket Guide to Chemical Hazards – Tantalum (metal and oxide dust, as Ta)|url = https://www.cdc.gov/niosh/npg/npgd0585.html|website = www.cdc.gov|accessdate = 2015-11-24}}</ref> |
|||
==তথ্যসূত্র== |
|||
{{reflist|30em}} |
|||
==বহিঃসংযোগ== |
|||
{{Wiktionary|tantalum}} |
|||
{{Commons|Tantalum}} |
|||
* [http://tanb.org/ Tantalum-Niobium International Study Center] |
|||
* [https://www.cdc.gov/niosh/npg/npgd0585.html CDC – NIOSH Pocket Guide to Chemical Hazards] |
|||
{{Clear}} |
|||
{{Compact periodic table}} |
|||
{{Tantalum compounds}} |
|||
{{Good article}} |
|||
{{Authority control}} |
|||
{{নিবিড় পর্যায় সারণী}} |
|||
[[বিষয়শ্রেণী:মৌলিক পদার্থ]] |
[[বিষয়শ্রেণী:মৌলিক পদার্থ]] |
||
[[বিষয়শ্রেণী:অবস্থান্তর ধাতু]] |
|||
'''ট্যানটালাম''' পর্যায় সারণীর ৭৩ নম্বর [[মৌল]], [[প্রতিক]] Ta। |
|||
১২:৫৫, ৬ জানুয়ারি ২০২০ তারিখে সংশোধিত সংস্করণ
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| সাধারণ বৈশিষ্ট্য | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| নাম, প্রতীক, পারমাণবিক সংখ্যা | ট্যানটালাম, Ta, ৭৩ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| রাসায়নিক শ্রেণী | অবস্থান্তর ধাতু | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| গ্রুপ, পর্যায়, ব্লক | ৫, ৬, ডি | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ভৌত রূপ | ধূসর নীল 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| পারমাণবিক ভর | ১৮০.৯৪৭৮৮(২) g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ইলেক্ট্রন বিন্যাস | [Xe] ৪এফ১৪ ৫ডি৩ ৬এস২ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| প্রতি শক্তিস্তরে ইলেকট্রন সংখ্যা | ২, ৮, ১৮, ৩২, ১১, ২ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ভৌত বৈশিষ্ট্য | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| দশা | কঠিন | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ঘনত্ব (সাধারণ তাপ ও চাপে) | ১৬.৬৯ g/cm³ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| গলনাংকে তরল ঘনত্ব | ১৫ গ্রাম/সেমি³ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| গলনাঙ্ক | ৩২৯০ K (৩০১৭ °C, ৫৪৬৩ °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| স্ফুটনাঙ্ক | ৫৭৩১ K (৫৪৫৮ °C, ৯৮৫৬ °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| গলনের লীন তাপ | ৩৬.৫৭ kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| বাষ্পীভবনের লীন তাপ | ৭৩২.৮ kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| তাপধারণ ক্ষমতা | (২৫ °সে) ২৫.৩৬ জুল/(মোল·কে) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| পারমাণবিক বৈশিষ্ট্য | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| কেলাসীয় গঠন | ঘনক কেন্দ্রিক | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| জারণ অবস্থা | 5 (হালকা আম্লিক অক্সাইড) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| তড়িৎ ঋণাত্মকতা | ১.৫ (পাউলিং স্কেল) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: ৭৬১ kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: ১৫০০ kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| পারমাণবিক ব্যাসার্ধ | ১৪৫ pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | ২০০ pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | ১৩৮ pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| অন্যান্য বৈশিষ্ট্য | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | কোন তথ্য নেই | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) ১৩১ nΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| তাপ পরিবাহিতা | (300 K) ৫৭.৫ W/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) ৬.৩ µm/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) ৩৪০০ m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ইয়ং এর গুণাঙ্ক | ১৮৬ GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | ৬৯ GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | ২০০ GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | ০.৩৪ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | ৬.৫ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | ৮৭৩ MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | ৮০০ MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| সি এ এস নিবন্ধন সংখ্যা | ৭৪৪০-২৫-৭ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| কয়েকটি উল্লেখযোগ্য সমস্থানিক | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ট্যানটালাম হল একটি মৌলিক পদার্থ, এর প্রতীক Ta এবং পারমাণবিক সংখ্যা ৭৩। আগে এর নাম ছিল ট্যানটালিয়াম , গ্রীক পৌরাণিক কাহিনীর খলনায়ক ট্যান্টালাসের নামে এখন এর নামকরণ করা হয়েছে।[১] ট্যানটালাম একটি বিরল, শক্ত, ধূসর নীল, [[দীপ্তি (খনিজ পদার্থ) |চকচকে)] অবস্থান্তর ধাতু, এটি ভীষণভাবে ক্ষয়-প্রতিরোধী। এটি উচ্চতাপ সহনশীল ধাতু দলের একটি অংশ, যেগুলি সঙ্কর ধাতু তৈরিতে ক্ষুদ্র উপাদান হিসাবে ব্যাপকভাবে ব্যবহৃত হয়। ট্যানটালাম রাসায়নিকভাবে নিষ্ক্রিয় হওয়ায়, পরীক্ষাগারে এটি একটি অত্যন্ত প্রয়োজনীয় মূল্যবান পদার্থ এবং এটিকে প্লাটিনামের বিকল্প হিসেবে ব্যবহার করা হয়। ট্যানটালাম ক্যাপাসিটার হিসেবে এটি প্রধানত ব্যবহার হয় বৈদ্যুতিন সরঞ্জামগুলিতে, যার মধ্যে আছে মোবাইল ফোন, ডিভিডি প্লেয়ার, ভিডিও গেইম কনসোল এবং ব্যক্তিগত কম্পিউটার। রাসায়নিকভাবে অনুরূপ নাইওবিয়ামের সাথে, ট্যানটালামকে সর্বদা ট্যানটালাইট, কলম্বাইট এবং কোল্টান খনিজ গ্রুপে (পৃথক খনিজ প্রজাতি হিসাবে স্বীকৃত না হলেও কলম্বাইট এবং ট্যানটালাইটের মিশ্রণ) দেখা যায়।[২] Tantalum is considered a technology-critical element.
ইতিহাস
১৮০২ সালে ট্যানটালাম আবিষ্কার করেছিলেন সুইডেনের অ্যান্ডার্স একেবার্গ, সুইডেন এবং ফিনল্যান্ডের দুটি খনিজ নমুনায় তিনি এর সন্ধান পান।[৩][৪] এর এক বছর আগে, চার্লস হ্যাচেট কলম্বিয়াম (এখন নাইওবিয়াম) আবিষ্কার করেছিলেন,[৫] এবং ১৮০৯ সালে ইংরেজ রসায়নবিদ উইলিয়াম হাইড ওল্লাস্টন এর অক্সাইড কলাম্বাইটের সাথে তুলনা করেছিলেন, with a density of 5.918 g/cm3, to that of tantalum, tantalite with a density of 7.935 g/cm3. He concluded that the two oxides, despite their difference in measured density, were identical and kept the name tantalum.[৬] After Friedrich Wöhler confirmed these results, it was thought that columbium and tantalum were the same element. This conclusion was disputed in 1846 by the German chemist Heinrich Rose, who argued that there were two additional elements in the tantalite sample, and he named them after the children of Tantalus: niobium (from Niobe, the goddess of tears), and pelopium (from Pelops).[৭][৮] The supposed element "pelopium" was later identified as a mixture of tantalum and niobium, and it was found that the niobium was identical to the columbium already discovered in 1801 by Hatchett.
The differences between tantalum and niobium were demonstrated unequivocally in 1864 by Christian Wilhelm Blomstrand,[৯] and Henri Etienne Sainte-Claire Deville, as well as by Louis J. Troost, who determined the empirical formulas of some of their compounds in 1865.[৯][১০] Further confirmation came from the Swiss chemist Jean Charles Galissard de Marignac,[১১] in 1866, who proved that there were only two elements. These discoveries did not stop scientists from publishing articles about the so-called ilmenium until 1871.[১২] De Marignac was the first to produce the metallic form of tantalum in 1864, when he reduced tantalum chloride by heating it in an atmosphere of hydrogen.[১৩] Early investigators had only been able to produce impure tantalum, and the first relatively pure ductile metal was produced by Werner von Bolton in Charlottenburg in 1903. Wires made with metallic tantalum were used for light bulb filaments until tungsten replaced it in widespread use.[১৪]
The name tantalum was derived from the name of the mythological Tantalus, the father of Niobe in Greek mythology. In the story, he had been punished after death by being condemned to stand knee-deep in water with perfect fruit growing above his head, both of which eternally tantalized him. (If he bent to drink the water, it drained below the level he could reach, and if he reached for the fruit, the branches moved out of his grasp.)[১৫] Anders Ekeberg wrote "This metal I call tantalum ... partly in allusion to its incapacity, when immersed in acid, to absorb any and be saturated."[১৬]
For decades, the commercial technology for separating tantalum from niobium involved the fractional crystallization of potassium heptafluorotantalate away from potassium oxypentafluoroniobate monohydrate, a process that was discovered by Jean Charles Galissard de Marignac in 1866. This method has been supplanted by solvent extraction from fluoride-containing solutions of tantalum.[১০]
Characteristics
Physical properties
Tantalum is dark (blue-gray),[১৭] dense, ductile, very hard, easily fabricated, and highly conductive of heat and electricity. The metal is renowned for its resistance to corrosion by acids; in fact, at temperatures below 150 °C tantalum is almost completely immune to attack by the normally aggressive aqua regia. It can be dissolved with hydrofluoric acid or acidic solutions containing the fluoride ion and sulfur trioxide, as well as with a solution of potassium hydroxide. Tantalum's high melting point of 3017 °C (boiling point 5458 °C) is exceeded among the elements only by tungsten, rhenium and osmium for metals, and carbon.
Tantalum exists in two crystalline phases, alpha and beta. The alpha phase is relatively ductile and soft; it has body-centered cubic structure (space group Im3m, lattice constant a = 0.33058 nm), Knoop hardness 200–400 HN and electrical resistivity 15–60 µΩ⋅cm. The beta phase is hard and brittle; its crystal symmetry is tetragonal (space group P42/mnm, a = 1.0194 nm, c = 0.5313 nm), Knoop hardness is 1000–1300 HN and electrical resistivity is relatively high at 170–210 µΩ⋅cm. The beta phase is metastable and converts to the alpha phase upon heating to 750–775 °C. Bulk tantalum is almost entirely alpha phase, and the beta phase usually exists as thin films[১৮] obtained by magnetron sputtering, chemical vapor deposition or electrochemical deposition from an eutectic molten salt solution.[১৯]
Isotopes
Natural tantalum consists of two isotopes: 180mTa (0.012%) and 181Ta (99.988%). 181Ta is a stable isotope. 180mTa (m denotes a metastable state) is predicted to decay in three ways: isomeric transition to the ground state of 180Ta, beta decay to 180W, or electron capture to 180Hf. However, radioactivity of this nuclear isomer has never been observed, and only a lower limit on its half-life of 2.0 × 1016 years has been set.[২০] The ground state of 180Ta has a half-life of only 8 hours. 180mTa is the only naturally occurring nuclear isomer (excluding radiogenic and cosmogenic short-lived nuclides). It is also the rarest primordial isotope in the Universe, taking into account the elemental abundance of tantalum and isotopic abundance of 180mTa in the natural mixture of isotopes (and again excluding radiogenic and cosmogenic short-lived nuclides).[২১]
Tantalum has been examined theoretically as a "salting" material for nuclear weapons (cobalt is the better-known hypothetical salting material). An external shell of 181Ta would be irradiated by the intensive high-energy neutron flux from a hypothetical exploding nuclear weapon. This would transmute the tantalum into the radioactive isotope 182Ta, which has a half-life of 114.4 days and produces gamma rays with approximately 1.12 million electron-volts (MeV) of energy apiece, which would significantly increase the radioactivity of the nuclear fallout from the explosion for several months. Such "salted" weapons have never been built or tested, as far as is publicly known, and certainly never used as weapons.[২২]
Tantalum can be used as a target material for accelerated proton beams for the production of various short-lived isotopes including 8Li, 80Rb, and 160Yb.[২৩]
Chemical compounds
Tantalum forms compounds in oxidation states −III to +V. Most commonly encountered are oxides of Ta(V), which includes all minerals. The chemical properties of Ta and Nb are very similar. In aqueous media, Ta only exhibit the +V oxidation state. Like niobium, tantalum is barely soluble in dilute solutions of hydrochloric, sulfuric, nitric and phosphoric acids due to the precipitation of hydrous Ta(V) oxide.[২৪] In basic media, Ta can be solubilized due to the formation of polyoxotantalate species.[২৫]
Oxides, nitrides, carbides, sulfides
Tantalum pentoxide (Ta2O5) is the most important compound from the perspective of applications. Oxides of tantalum in lower oxidation states are numerous, including many defect structures, and are lightly studied or poorly characterized.[২৬]
Tantalates, compounds containing [TaO4]3− or [TaO3]− are numerous. Lithium tantalate (LiTaO3) adopts a perovskite structure. Lanthanum tantalate (LaTaO4) contains isolated TaO3−
4 tetrahedra.[২৭]
As in the cases of other refractory metals, the hardest known compounds of tantalum are nitrides and carbides. Tantalum carbide, TaC, like the more commonly used tungsten carbide, is a hard ceramic that is used in cutting tools. Tantalum(III) nitride is used as a thin film insulator in some microelectronic fabrication processes.[২৮]
The best studied chalcogenide is TaS2, a layered semiconductor, as seen for other transition metal dichalcogenides. A tantalum-tellurium alloy forms quasicrystals.[২৭]
Halides
Tantalum halides span the oxidation states of +5, +4, and +3. Tantalum pentafluoride (TaF5) is a white solid with a melting point of 97.0 °C. The anion [TaF7]2- is used for its separation from niobium.[২৯] The chloride TaCl
5, which exists as a dimer, is the main reagent in synthesis of new Ta compounds. It hydrolyzes readily to an oxychloride. The lower halides TaX
4 and TaX
3, feature Ta-Ta bonds.[২৭][২৪][২৭]
Organotantalum compounds
Organotantalum compounds include pentamethyltantalum, mixed alkyltantalum chlorides, alkyltantalum hydrides, alkylidene complexes as well as cyclopentadienyl derivatives of the same.[৩০][৩১] Diverse salts and substituted derivatives are known for the hexacarbonyl [Ta(CO)6]− and related isocyanides.

Occurrence

Tantalum is estimated to make up about 1 ppm[৩২] or 2 ppm[২৪] of the Earth's crust by weight. There are many species of tantalum minerals, only some of which are so far being used by industry as raw materials: tantalite (a series consisting of tantalite-(Fe), tantalite-(Mn) and tantalite-(Mg)) microlite (now a group name), wodginite, euxenite (actually euxenite-(Y)), and polycrase (actually polycrase-(Y)).[২] Tantalite (Fe, Mn)Ta2O6 is the most important mineral for tantalum extraction. Tantalite has the same mineral structure as columbite (Fe, Mn) (Ta, Nb)2O6; when there is more tantalum than niobium it is called tantalite and when there is more niobium than tantalum is it called columbite (or niobite). The high density of tantalite and other tantalum containing minerals makes the use of gravitational separation the best method. Other minerals include samarskite and fergusonite.
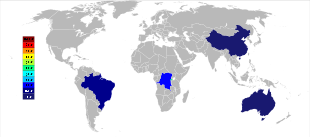
The primary mining of tantalum is in Australia, where the largest producer, Global Advanced Metals, formerly known as Talison Minerals, operates two mines in Western Australia, Greenbushes in the Southwest and Wodgina in the Pilbara region. The Wodgina mine was reopened in January 2011 after mining at the site was suspended in late-2008 due to the global financial crisis.[৩৩] Less than a year after it reopened, Global Advanced Metals announced that due to again "... softening tantalum demand ...", and other factors, tantalum mining operations were to cease at the end of February 2012.[৩৪] Wodgina produces a primary tantalum concentrate which is further upgraded at the Greenbushes operation before being sold to customers.[৩৫] Whereas the large-scale producers of niobium are in Brazil and Canada, the ore there also yields a small percentage of tantalum. Some other countries such as China, Ethiopia, and Mozambique mine ores with a higher percentage of tantalum, and they produce a significant percentage of the world's output of it. Tantalum is also produced in Thailand and Malaysia as a by-product of the tin mining there. During gravitational separation of the ores from placer deposits, not only is cassiterite (SnO2) found, but a small percentage of tantalite also included. The slag from the tin smelters then contains economically useful amounts of tantalum, which is leached from the slag.[১০][৩৬]
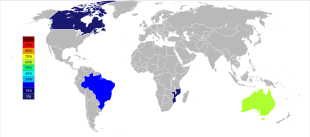
World tantalum mine production has undergone an important geographic shift since the start of the 21st century when production was predominantly from Australia and Brazil. Beginning in 2007 and through 2014, the major sources of tantalum production from mines dramatically shifted to the DRC, Rwanda, and some other African countries.[৩৭] Future sources of supply of tantalum, in order of estimated size, are being explored in Saudi Arabia, Egypt, Greenland, China, Mozambique, Canada, Australia, the United States, Finland, and Brazil.[৩৮][৩৯]
It is estimated that there are less than 50 years left of tantalum resources, based on extraction at current rates, demonstrating the need for increased recycling.[৪০]
Status as a conflict resource
Tantalum is considered a conflict resource. Coltan, the industrial name for a columbite–tantalite mineral from which niobium and tantalum are extracted,[৪১] can also be found in Central Africa, which is why tantalum is being linked to warfare in the Democratic Republic of the Congo (formerly Zaire). According to an October 23, 2003 United Nations report,[৪২] the smuggling and exportation of coltan has helped fuel the war in the Congo, a crisis that has resulted in approximately 5.4 million deaths since 1998[৪৩] – making it the world’s deadliest documented conflict since World War II. Ethical questions have been raised about responsible corporate behavior, human rights, and endangering wildlife, due to the exploitation of resources such as coltan in the armed conflict regions of the Congo Basin.[৪৪][৪৫][৪৬][৪৭] However, although important for the local economy in Congo, the contribution of coltan mining in Congo to the world supply of tantalum is usually small. The United States Geological Survey reports in its yearbook that this region produced a little less than 1% of the world's tantalum output in 2002–2006, peaking at 10% in 2000 and 2008.[৩৬]
The stated aim of the Solutions for Hope Tantalum Project is to "source conflict-free tantalum from the Democratic Republic of Congo"[৪৮]
Production and fabrication

Several steps are involved in the extraction of tantalum from tantalite. First, the mineral is crushed and concentrated by gravity separation. This is generally carried out near the mine site.
Refining
The refining of tantalum from its ores is one of the more demanding separation processes in industrial metallurgy. The chief problem is that tantalum ores contain significant amounts of niobium, which has chemical properties almost identical to those of Ta. A large number of procedures have been developed to address this challenge.
In modern times, the separation is achieved by hydrometallurgy.[৫০] Extraction begins with leaching the ore with hydrofluoric acid together with sulfuric acid or hydrochloric acid. This step allows the tantalum and niobium to be separated from the various non-metallic impurities in the rock. Although Ta occurs as various minerals, it is conveniently represented as the pentoxide, since most oxides of tantalum(V) behave similarly under these conditions. A simplified equation for its extraction is thus:
- Ta2O5 + 14 HF → 2 H2[TaF7] + 5 H2O
Completely analogous reactions occur for the niobium component, but the hexafluoride is typically predominant under the conditions of the extraction.
- Nb2O5 + 12 HF → 2 H[NbF6] + 5 H2O
These equations are simplified: it is suspected that bisulfate (HSO4−) and chloride compete as ligands for the Nb(V) and Ta(V) ions, when sulfuric and hydrochloric acids are used, respectively.[৫০] The tantalum and niobium fluoride complexes are then removed from the aqueous solution by liquid-liquid extraction into organic solvents, such as cyclohexanone, octanol, and methyl isobutyl ketone. This simple procedure allows the removal of most metal-containing impurities (e.g. iron, manganese, titanium, zirconium), which remain in the aqueous phase in the form of their fluorides and other complexes.
Separation of the tantalum from niobium is then achieved by lowering the ionic strength of the acid mixture, which causes the niobium to dissolve in the aqueous phase. It is proposed that oxyfluoride H2[NbOF5] is formed under these conditions. Subsequent to removal of the niobium, the solution of purified H2[TaF7] is neutralised with aqueous ammonia to precipitate hydrated tantalum oxide as a solid, which can be calcined to tantalum pentoxide (Ta2O5).[৫১]
Instead of hydrolysis, the H2[TaF7] can be treated with potassium fluoride to produce potassium heptafluorotantalate:
- H2[TaF7] + 2 KF → K2[TaF7] + 2 HF
Unlike H2[TaF7], the potassium salt is readily crystallized and handled as a solid.
K2[TaF7] can be converted to metallic tantalum by reduction with sodium, at approximately 800 °C in molten salt.[৫২]
- K2[TaF7] + 5 Na → Ta + 5 NaF + 2 KF
In an older method, called the Marignac process, the mixture of H2[TaF7] and H2[NbOF5] was converted to a mixture of K2[TaF7] and K2[NbOF5], which was then be separated by fractional crystallization, exploiting their different water solubilities.
Electrolysis
Tantalum can also be refined by electrolysis, using a modified version of the Hall–Héroult process. Instead of requiring the input oxide and output metal to be in liquid form, tantalum electrolysis operates on non-liquid powdered oxides. The initial discovery came in 1997 when Cambridge University researchers immersed small samples of certain oxides in baths of molten salt and reduced the oxide with electric current. The cathode uses powdered metal oxide. The anode is made of carbon. The molten salt at ১,০০০ °সে (১,৮৩০ °ফা) is the electrolyte. The first refinery has enough capacity to supply 3–4% of annual global demand.[৫৩]
Fabrication and metalworking
All welding of tantalum must be done in an inert atmosphere of argon or helium in order to shield it from contamination with atmospheric gases. Tantalum is not solderable. Grinding tantalum is difficult, especially so for annealed tantalum. In the annealed condition, tantalum is extremely ductile and can be readily formed as metal sheets.[৫৪]
Applications
Electronics
The major use for tantalum, as the metal powder, is in the production of electronic components, mainly capacitors and some high-power resistors. Tantalum electrolytic capacitors exploit the tendency of tantalum to form a protective oxide surface layer, using tantalum powder, pressed into a pellet shape, as one "plate" of the capacitor, the oxide as the dielectric, and an electrolytic solution or conductive solid as the other "plate". Because the dielectric layer can be very thin (thinner than the similar layer in, for instance, an aluminium electrolytic capacitor), a high capacitance can be achieved in a small volume. Because of the size and weight advantages, tantalum capacitors are attractive for portable telephones, personal computers, automotive electronics and cameras.[৫৫]
Alloys
Tantalum is also used to produce a variety of alloys that have high melting points, strength, and ductility. Alloyed with other metals, it is also used in making carbide tools for metalworking equipment and in the production of superalloys for jet engine components, chemical process equipment, nuclear reactors, missile parts, heat exchangers, tanks, and vessels.[৫৬][৫৫][৫৭] Because of its ductility, tantalum can be drawn into fine wires or filaments, which are used for evaporating metals such as aluminium. Since it resists attack by body fluids and is nonirritating, tantalum is widely used in making surgical instruments and implants. For example, porous tantalum coatings are used in the construction of orthopedic implants due to tantalum's ability to form a direct bond to hard tissue.[৫৮]
Tantalum is inert against most acids except hydrofluoric acid and hot sulfuric acid, and hot alkaline solutions also cause tantalum to corrode. This property makes it a useful metal for chemical reaction vessels and pipes for corrosive liquids. Heat exchanging coils for the steam heating of hydrochloric acid are made from tantalum.[৫৯] Tantalum was extensively used in the production of ultra high frequency electron tubes for radio transmitters. Tantalum is capable of capturing oxygen and nitrogen by forming nitrides and oxides and therefore helped to sustain the high vacuum needed for the tubes when used for internal parts such as grids and plates.[২৯][৫৯]
Other uses

The high melting point and oxidation resistance lead to the use of the metal in the production of vacuum furnace parts. Tantalum is extremely inert and is therefore formed into a variety of corrosion resistant parts, such as thermowells, valve bodies, and tantalum fasteners. Due to its high density, shaped charge and explosively formed penetrator liners have been constructed from tantalum.[৬০] Tantalum greatly increases the armor penetration capabilities of a shaped charge due to its high density and high melting point.[৬১][৬২] It is also occasionally used in precious watches e.g. from Audemars Piguet, F.P. Journe, Hublot, Montblanc, Omega, and Panerai. Tantalum is also highly bioinert and is used as an orthopedic implant material.[৬৩] The high stiffness of tantalum makes it necessary to use it as highly porous foam or scaffold with lower stiffness for hip replacement implants to avoid stress shielding.[৬৪] Because tantalum is a non-ferrous, non-magnetic metal, these implants are considered to be acceptable for patients undergoing MRI procedures.[৬৫] The oxide is used to make special high refractive index glass for camera lenses.[৬৬]
Environmental issues
Tantalum receives far less attention in the environmental field than it does in other geosciences. Upper Crust Concentrations (UCC) and the Nb/Ta ratio in the upper crust and in minerals are available because these measurements are useful as a geochemical tool.[৬৭] The latest values for UCC and the Nb/Ta(w/w) ratio in the upper crust stand at 0.92 ppm and 12.7 respectively.[৬৮]
Little data is available on tantalum concentrations in the different environmental compartments, especially in natural waters where reliable estimates of ‘dissolved’ tantalum concentrations in seawater and freshwaters have not even been produced.[৬৯] Some values on dissolved concentrations in oceans have been published, but they are contradictory. Values in freshwaters fare little better, but, in all cases, they are probably below 1 ng L−1,since ‘dissolved’ concentrations in natural waters are well below most current analytical capabilities.[৭০] Analysis requires pre-concentration procedures that, for the moment, do not give consistent results. And in any case, tantalum appears to be present in natural waters mostly as particulate matter rather than dissolved.[৬৯]
Values for concentrations in soils, bed sediments and atmospheric aerosols are easier to come by.[৬৯] Values in soils are close to 1 ppm and thus to UCC values. This indicates detrital origin. For atmospheric aerosols the values available are scattered and limited. When tantalum enrichment is observed, it is probably due to loss of more water-soluble elements in aerosols in the clouds.[৭১]
Pollution linked to human use of the element has not been detected.[৭২] Tantalum appears to be a very conservative element in biogeochemical terms, but its cycling and reactivity are still not fully understood.
Precautions
Compounds containing tantalum are rarely encountered in the laboratory. The metal is highly biocompatible[৬৩] and is used for body implants and coatings, therefore attention may be focused on other elements or the physical nature of the chemical compound.[৭৩]
People can be exposed to tantalum in the workplace by breathing it in, skin contact, or eye contact. The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for tantalum exposure in the workplace as 5 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 5 mg/m3 over an 8-hour workday and a short-term limit of 10 mg/m3. At levels of 2500 mg/m3, tantalum is immediately dangerous to life and health.[৭৪]
তথ্যসূত্র
- ↑ Euripides, Orestes
- ↑ ক খ "Mindat.org - Mines, Minerals and More"। www.mindat.org।
- ↑ Ekeberg, Anders (১৮০২)। "Of the Properties of the Earth Yttria, compared with those of Glucine; of Fossils, in which the first of these Earths in contained; and of the Discovery of a metallic Nature (Tantalium)"। Journal of Natural Philosophy, Chemistry, and the Arts। 3: 251–255।
- ↑ Ekeberg, Anders (১৮০২)। "Uplysning om Ytterjorden egenskaper, i synnerhet i aemforelse med Berylljorden:om de Fossilier, havari förstnemnde jord innehales, samt om en ny uptäckt kropp af metallik natur"। Kungliga Svenska Vetenskapsakademiens Handlingar। 23: 68–83।
- ↑ Griffith, William P.; Morris, Peter J. T. (২০০৩)। "Charles Hatchett FRS (1765–1847), Chemist and Discoverer of Niobium"। Notes and Records of the Royal Society of London। 57 (3): 299–316। জেস্টোর 3557720। ডিওআই:10.1098/rsnr.2003.0216।
- ↑ Wollaston, William Hyde (১৮০৯)। "On the Identity of Columbium and Tantalum"। Philosophical Transactions of the Royal Society of London। 99: 246–252। জেস্টোর 107264। ডিওআই:10.1098/rstl.1809.0017।
- ↑ Rose, Heinrich (১৮৪৪)। "Ueber die Zusammensetzung der Tantalite und ein im Tantalite von Baiern enthaltenes neues Metall"। Annalen der Physik (German ভাষায়)। 139 (10): 317–341। ডিওআই:10.1002/andp.18441391006। বিবকোড:1844AnP...139..317R।
- ↑ Rose, Heinrich (১৮৪৭)। "Ueber die Säure im Columbit von Nordamérika"। Annalen der Physik (German ভাষায়)। 146 (4): 572–577। ডিওআই:10.1002/andp.18471460410। বিবকোড:1847AnP...146..572R।
- ↑ ক খ Marignac, Blomstrand; H. Deville; L. Troost & R. Hermann (১৮৬৬)। "Tantalsäure, Niobsäure, (Ilmensäure) und Titansäure"। Fresenius' Journal of Analytical Chemistry। 5 (1): 384–389। ডিওআই:10.1007/BF01302537।
- ↑ ক খ গ Gupta, C. K.; Suri, A. K. (১৯৯৪)। Extractive Metallurgy of Niobium। CRC Press। আইএসবিএন 978-0-8493-6071-8।
- ↑ Marignac, M. C. (১৮৬৬)। "Recherches sur les combinaisons du niobium"। Annales de Chimie et de Physique (French ভাষায়)। 4 (8): 7–75।
- ↑ Hermann, R. (১৮৭১)। "Fortgesetzte Untersuchungen über die Verbindungen von Ilmenium und Niobium, sowie über die Zusammensetzung der Niobmineralien (Further research about the compounds of ilmenium and niobium, as well as the composition of niobium minerals)"। Journal für Praktische Chemie (German ভাষায়)। 3 (1): 373–427। ডিওআই:10.1002/prac.18710030137।
- ↑ "Niobium"। Universidade de Coimbra। সংগ্রহের তারিখ ২০০৮-০৯-০৫।
- ↑ Bowers, B. (২০০১)। "Scanning Our Past from London The Filament Lamp and New Materials"। Proceedings of the IEEE। 89 (3): 413। ডিওআই:10.1109/5.915382।
- ↑ Aycan, Sule (২০০৫)। "Chemistry Education and Mythology" (পিডিএফ)। Journal of Social Sciences। 1 (4): 238–239। ডিওআই:10.3844/jssp.2005.238.239।
- ↑ Greenwood, N. N.; Earnshaw, A. (১৯৯৭)। Chemistry of the Elements (2nd সংস্করণ)। Butterworth-Heinemann। পৃষ্ঠা 1138। আইএসবিএন 0080379419।
- ↑ Colakis, Marianthe; Masello, Mary Joan (২০০৭-০৬-৩০)। "Tantalum"। Classical Mythology & More: A Reader Workbook। আইএসবিএন 978-0-86516-573-1।
- ↑ Magnuson, M.; Greczynski, G.; Eriksson, F.; Hultman, L.; Hogberg, H. (২০১৯)। "Electronic structure of β-Ta films from X-ray photoelectron spectroscopy and first-principles calculations"। Applied Surface Science। 470: 607–612। ডিওআই:10.1016/j.apsusc.2018.11.096।
- ↑ Lee, S.; Doxbeck, M.; Mueller, J.; Cipollo, M.; Cote, P. (২০০৪)। "Texture, structure and phase transformation in sputter beta tantalum coating"। Surface and Coatings Technology। 177–178: 44। ডিওআই:10.1016/j.surfcoat.2003.06.008।
- ↑ Hult, Mikael; Wieslander, J. S. Elisabeth; Marissens, Gerd; Gasparro, Joël; Wätjen, Uwe; Misiaszek, Marcin (২০০৯)। "Search for the radioactivity of 180mTa using an underground HPGe sandwich spectrometer"। Applied Radiation and Isotopes। 67 (5): 918–921। ডিওআই:10.1016/j.apradiso.2009.01.057। পিএমআইডি 19246206।
- ↑ আউডি, জর্জেস; বার্সিলন, অলিভিয়ের; ব্লাকহট, জিন; ওয়াপস্ট্রা, অল্ডার্ট হেনড্রিক (২০০৩), "The NUBASE evaluation of nuclear and decay properties" [পারমাণবিক এবং ক্ষয় বৈশিষ্ট্যের নুবেস মূল্যায়ন], নিউক্লিয়ার ফিজিক্স এ (ইংরেজি ভাষায়), ৭২৯: ৩–১২৮, ডিওআই:10.1016/j.nuclphysa.2003.11.001, বিবকোড:2003NuPhA.729....3A
- ↑ Win, David Tin; Al Masum, Mohammed (২০০৩)। "Weapons of Mass Destruction" (পিডিএফ)। Assumption University Journal of Technology। 6 (4): 199–219।
- ↑ "Tantalum Target Yields - ISAC Yield Database - TRIUMF : Canada's National Laboratory for Particle and Nuclear Physics"। mis.triumf.ca।
- ↑ ক খ গ Agulyansky, Anatoly (২০০৪)। The Chemistry of Tantalum and Niobium Fluoride Compounds। Elsevier। আইএসবিএন 978-0-444-51604-6। সংগ্রহের তারিখ ২০০৮-০৯-০২।
- ↑ Deblonde, Gauthier J. -P.; Chagnes, Alexandre; Bélair, Sarah; Cote, Gérard (২০১৫-০৭-০১)। "Solubility of niobium(V) and tantalum(V) under mild alkaline conditions"। Hydrometallurgy। 156: 99–106। আইএসএসএন 0304-386X। ডিওআই:10.1016/j.hydromet.2015.05.015।
- ↑ Greenwood, N. N.; Earnshaw, A. (১৯৯৭)। Chemistry of the Elements (2nd সংস্করণ)। Butterworth-Heinemann। আইএসবিএন 0080379419।
- ↑ ক খ গ ঘ Holleman, A. F.; Wiberg, E.; Wiberg, N. (২০০৭)। Lehrbuch der Anorganischen Chemie (German ভাষায়) (102nd সংস্করণ)। de Gruyter। আইএসবিএন 978-3-11-017770-1।
- ↑ Tsukimoto, S.; Moriyama, M.; Murakami, Masanori (১৯৬১)। "Microstructure of amorphous tantalum nitride thin films"। Thin Solid Films। 460 (1–2): 222–226। ডিওআই:10.1016/j.tsf.2004.01.073। বিবকোড:2004TSF...460..222T।
- ↑ ক খ Soisson, Donald J.; McLafferty, J. J.; Pierret, James A. (১৯৬১)। "Staff-Industry Collaborative Report: Tantalum and Niobium"। Ind. Eng. Chem.। 53 (11): 861–868। ডিওআই:10.1021/ie50623a016।
- ↑ Schrock, Richard R. (১৯৭৯-০৩-০১)। "Alkylidene complexes of niobium and tantalum"। Accounts of Chemical Research। 12 (3): 98–104। আইএসএসএন 0001-4842। ডিওআই:10.1021/ar50135a004।
- ↑ Morse, P. M.; ও অন্যান্য (২০০৮)। "Ethylene Complexes of the Early Transition Metals: Crystal Structures of [HfEt
4(C
2H
4)2−
] and the Negative-Oxidation-State Species [TaHEt(C
2H
4)3−
3] and [WH(C
2H
4)3−
4]"। Organometallics। 27 (5): 984। ডিওআই:10.1021/om701189e। - ↑ Emsley, John (২০০১)। "Tantalum"। Nature's Building Blocks: An A-Z Guide to the Elements। Oxford, England, UK: Oxford University Press। পৃষ্ঠা 420। আইএসবিএন 978-0-19-850340-8।
- ↑ "Talison Tantalum eyes mid-2011 Wodgina restart 2010-06-09"। Reuters। ২০১০-০৬-০৯। সংগ্রহের তারিখ ২০১০-০৮-২৭।
- ↑ Emery, Kate (২৪ জানু ২০১২)। "GAM closes Wodgina tantalum mine"। The West Australian। ৪ ডিসেম্বর ২০১২ তারিখে মূল থেকে আর্কাইভ করা। সংগ্রহের তারিখ ২০ মার্চ ২০১২।
Worldwide softening tantalum demand and delays in receiving Governmental approval for installation of necessary crushing equipment are among contributing factors in this decision
- ↑ "Wodgina Operations"। Global Advanced Metals। ২০০৮। সংগ্রহের তারিখ ২০১১-০৩-২৮।
- ↑ ক খ Papp, John F. (২০০৬)। "2006 Minerals Yearbook Nb & Ta"। US Geological Survey। সংগ্রহের তারিখ ২০০৮-০৬-০৩।
- ↑ Bleiwas, Donald I.; Papp, John F.; Yager, Thomas R. (২০১৫)। "Shift in Global Tantalum Mine Production, 2000–2014" (পিডিএফ)। U.S. Geological Survey।
- ↑ M. J. (নভেম্বর ২০০৭)। "Tantalum supplement" (পিডিএফ)। Mining Journal। সংগ্রহের তারিখ ২০০৮-০৬-০৩।
- ↑ "International tantalum resources — exploration and mining" (পিডিএফ)। GSWA Mineral Resources Bulletin। 22 (10)। ২০০৭-০৯-২৬ তারিখে মূল (পিডিএফ) থেকে আর্কাইভ করা।
- ↑ "How much is left?"। সংগ্রহের তারিখ ২০১৩-০১-১৩।
- ↑ Tantalum-Niobium International Study Center: Coltan Retrieved 2008-01-27
- ↑ "S/2003/1027"। ২০০৩-১০-২৬। সংগ্রহের তারিখ ২০০৮-০৪-১৯।
- ↑ "Special Report: Congo"। International Rescue Committee। সংগ্রহের তারিখ ২০০৮-০৪-১৯।
- ↑ Hayes, Karen; Burge, Richard (২০০৩)। Coltan Mining in the Democratic Republic of Congo: How tantalum-using industries can commit to the reconstruction of the DRC। Fauna & Flora। পৃষ্ঠা 1–64। আইএসবিএন 978-1-903703-10-6।
- ↑ Dizolele, Mvemba Phezo (জানুয়ারি ৬, ২০১১)। "Congo's Bloody Coltan"। Pulitzer Center on Crisis Reporting। সংগ্রহের তারিখ ২০০৯-০৮-০৮।
- ↑ "Congo War and the Role of Coltan"। ২০০৯-০৭-১৩ তারিখে মূল থেকে আর্কাইভ করা। সংগ্রহের তারিখ ২০০৯-০৮-০৮।
- ↑ "Coltan mining in the Congo River Basin"। ২০০৯-০৩-৩০ তারিখে মূল থেকে আর্কাইভ করা। সংগ্রহের তারিখ ২০০৯-০৮-০৮।
- ↑ "'Solutions for Hope' Tantalum Project Offers Solutions and Brings Hope to the People of the DRC"। Solutions Network। সংগ্রহের তারিখ ১৮ সেপ্টেম্বর ২০১৪।
- ↑ "Mineral Resources Program"। minerals.usgs.gov। জুন ৪, ২০১৩ তারিখে মূল থেকে আর্কাইভ করা।
- ↑ ক খ Zhaowu Zhu; Chu Yong Cheng (২০১১)। "Solvent extraction technology for the separation and purification of niobium and tantalum: A review"। Hydrometallurgy। 107 (1–2): 1–12। ডিওআই:10.1016/j.hydromet.2010.12.015।
- ↑ Agulyanski, Anatoly (২০০৪)। Chemistry of Tantalum and Niobium Fluoride Compounds (1st সংস্করণ)। Burlington: Elsevier। আইএসবিএন 9780080529028।
- ↑ Okabe, Toru H.; Sadoway, Donald R. (১৯৯৮)। "Metallothermic reduction as an electronically mediated reaction"। Journal of Materials Research। 13 (12): 3372–3377। ডিওআই:10.1557/JMR.1998.0459। বিবকোড:1998JMatR..13.3372O।
- ↑ "Manufacturing metals: A tantalising prospect"। The Economist। ২০১৩-০২-১৬। সংগ্রহের তারিখ ২০১৩-০৪-১৭।
- ↑ "NFPA 484 – Standard for Combustible Metals, Metal Powders, and Metal Dusts – 2002 Edition" (পিডিএফ)। National Fire Protection Association। NFPA। ২০০২-০৮-১৩। সংগ্রহের তারিখ ২০১৬-০২-১২।
- ↑ ক খ "Commodity Report 2008: Tantalum" (পিডিএফ)। United States Geological Survey। সংগ্রহের তারিখ ২০০৮-১০-২৪।
- ↑ "Tantalum Products: Tantalum Sheet & Plate | Admat Inc"। Admat Inc. (ইংরেজি ভাষায়)। সংগ্রহের তারিখ ২০১৮-০৮-২৮।
- ↑ Buckman Jr., R. W. (২০০০)। "New applications for tantalum and tantalum alloys"। JOM Journal of the Minerals, Metals and Materials Society। 52 (3): 40। ডিওআই:10.1007/s11837-000-0100-6। বিবকোড:2000JOM....52c..40B।
- ↑ Cohen, R.; Della Valle, C. J.; Jacobs, J. J. (২০০৬)। "Applications of porous tantalum in total hip arthroplasty"। Journal of the American Academy of Orthopaedic Surgeons। 14 (12): 646–55। ডিওআই:10.5435/00124635-200611000-00008। পিএমআইডি 17077337।
- ↑ ক খ Balke, Clarence W. (১৯৩৫)। "Columbium and Tantalum"। Industrial and Engineering Chemistry। 20 (10): 1166। ডিওআই:10.1021/ie50310a022।
- ↑ Nemat-Nasser, Sia; Isaacs, Jon B.; Liu, Mingqi (১৯৯৮)। "Microstructure of high-strain, high-strain-rate deformed tantalum"। Acta Materialia। 46 (4): 1307। ডিওআই:10.1016/S1359-6454(97)00746-5।
- ↑ Walters, William; Cooch, William; Burkins, Matthew; Burkins, Matthew (২০০১)। "The penetration resistance of a titanium alloy against jets from tantalum shaped charge liners"। International Journal of Impact Engineering। 26 (1–10): 823। ডিওআই:10.1016/S0734-743X(01)00135-X।
- ↑ Russell, Alan M.; Lee, Kok Loong (২০০৫)। Structure-property relations in nonferrous metals। Hoboken, NJ: Wiley-Interscience। পৃষ্ঠা 218। আইএসবিএন 978-0-471-64952-6।
- ↑ ক খ Gerald L. Burke (১৯৪০)। "The Corrosion of Metals in Tissues; and An Introduction to Tantalum"। Canadian Medical Association Journal। 43।
- ↑ Black, J. (১৯৯৪)। "Biological performance of tantalum"। Clinical Materials। 16 (3): 167–173। ডিওআই:10.1016/0267-6605(94)90113-9। পিএমআইডি 10172264।
- ↑ Paganias, Christos G.; Tsakotos, George A.; Koutsostathis, Stephanos D.; Macheras, George A. (২০১২)। "Osseous integration in porous tantalum implants"। Indian Journal of Orthopaedics। 46 (5): 505–13। আইএসএসএন 0019-5413। ডিওআই:10.4103/0019-5413.101032। পিএমআইডি 23162141। পিএমসি 3491782
 ।
।
- ↑ Musikant, Solomon (১৯৮৫)। "Optical Glass Composition"। Optical Materials: An Introduction to Selection and Application। CRC Press। পৃষ্ঠা 28। আইএসবিএন 978-0-8247-7309-0।
- ↑ Green, TH. (১৯৯৫)। "Significance of Nb/Ta as an indicator of geochemical processes in the crust-mantle system"। Chemical Geology। 120 (3–4): 347–359। ডিওআই:10.1016/0009-2541(94)00145-X। বিবকোড:1995ChGeo.120..347G।
- ↑ Hu, Z.; Gao, S. (২০০৮)। "Upper crustal abundances of trace elements: a revision and update"। Chemical Geology। 253 (3–4): 205। ডিওআই:10.1016/j.chemgeo.2008.05.010। বিবকোড:2008ChGeo.253..205H।
- ↑ ক খ গ Filella, M. (২০১৭)। "Tantalum in the environment"। Earth-Science Reviews। 173: 122–140। ডিওআই:10.1016/j.earscirev.2017.07.002।
- ↑ Filella, M.; Rodushkin, I. (২০১৮)। "A concise guide for the determination of less-studied technology-critical elements (Nb, Ta, Ga, In, Ge, Te) by inductively coupled plasma mass spectrometry in environmental samples"। Spectrochimica Acta Part B। 141: 80–84। ডিওআই:10.1016/j.sab.2018.01.004।
- ↑ Vlastelic, I.; Suchorski, K.; Sellegri, K.; Colomb, A.; Nauret, F.; Bouvier, L.; Piro, J-L. (২০১৫)। "The high field strength element budget of atmospheric aerosols (puy de Dôme, France)"। Geochimica et Cosmochimica Acta। 167: 253–268। ডিওআই:10.1016/j.gca.2015.07.006। বিবকোড:2015GeCoA.167..253V।
- ↑ Filella, M.; Rodríguez-Murillo, JC. (২০১৭)। "Less-studied TCE: are their environmental concentrations increasing due to their use in new technologies?"। Chemosphere। 182: 605–616। ডিওআই:10.1016/j.chemosphere.2017.05.024। পিএমআইডি 28525874।
- ↑ Matsuno H; Yokoyama A; Watari F; Uo M; Kawasaki T. (২০০১)। "Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biocompatibility of tantalum."। Biomaterials। 22 (11): 1253–62। ডিওআই:10.1016/S0142-9612(00)00275-1। পিএমআইডি 11336297।
- ↑ "CDC – NIOSH Pocket Guide to Chemical Hazards – Tantalum (metal and oxide dust, as Ta)"। www.cdc.gov। সংগ্রহের তারিখ ২০১৫-১১-২৪।
বহিঃসংযোগ

